The Second Law of Thermodynamics & Entropy
Last updated on January 7th, 2022 at 03:34 pm
To understand the Second Law of Thermodynamics it’s essential to understand the concept of Entropy. Once we get an idea of entropy with an example, we will discuss 3 different forms or statements of the 2nd law of thermodynamics.
[Readers also viewed these:
zeroth law of thermodynamics
and
first law of thermodynamics ]
Entropy or disorder
Let’s consider a box containing two pure gases separated by a partition. Now, if the partition is removed, the gases would mix, and the positions of the gas molecules would be random.
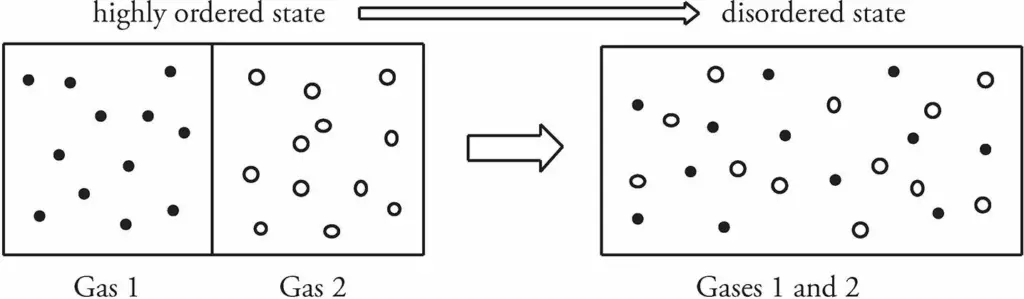
A closed system that shows a high degree of order tends to evolve in such a way that its degree of order decreases. In other words, disorder (or, as it’s technically called, entropy) increases.
If we started with the box on the right, containing the mixture of the gases, it would be virtually impossible that at any later time all the molecules of Gas 1 would happen to move to the left side of the box while all the molecules of Gas 2 spontaneously moved to the right side of the box.
In a way, the second law of thermodynamics defines the direction of time: Time flows in such a way that ordered systems become disordered.
Disordered states do not spontaneously become ordered without any other change taking place.
Second law of thermodynamics – in terms of disorder or entropy
The following is the essence of one form of the second law of thermodynamics:
The total amount of disorder—the total entropy—of a system plus its surroundings will never decrease.
A system always increases in entropy over time
Now, it is possible for the entropy of a system to decrease, but it’ll always be at the expense of a greater increase in entropy in the surroundings.
- For example, when water freezes, its entropy decreases. The molecules making up an ice crystal have a more structured order than the random collection of water molecules in the liquid phase, so the entropy of the water decreases when it freezes.
- But when water freezes, it releases heat energy into its environment, which creates disorder in the surroundings.
- If we were to figure out the total change in entropy of the water plus its surroundings, we’d find that although the entropy of the water itself decreased, it was more than compensated by a greater amount of entropy increase in the surroundings.
- So, the total entropy of the system and its surroundings increased, in agreement with the second law of thermodynamics.
Entropy and Heat – A system always increases in entropy over time. This entropy is usually in the form of heat given off.
Equivalent statements of the second law
There are several equivalent statements of the second law.
- In addition to the entropy form, another form says that heat always flows from an object at a higher temperature to an object at a lower temperature, never the other way around. Heat always flows from hot to cold, never cold to hot.
- Another form, says that a heat engine can never operate at 100% efficiency, or, equivalently, that it is impossible to convert heat completely into work.
Related study: Read about Concepts of Heat Engine
