Heat capacity & Specific heat capacity – explanation & measurement
In this post, we will first go for the definitions, formulas, units, and explanations of Specific Heat Capacity & Heat Capacity. Then we will see how to measure Specific heat capacity.
- Definition & formula of Heat Capacity or thermal capacity
- Definition & formula of Specific heat capacity
- specific heat capacity – Examples & values
- Explanation of different Heat capacity & Specific heat capacity of different materials
- Methods of measuring Specific heat capacity
- Calculation of energy using the formulas
Definition & formula of Heat Capacity or thermal capacity
We define the thermal capacity C of an object as the energy required to raise its temperature by 1 K. Different objects (even different samples of the same substance) will have different values of heat capacity.
The formula of Heat Capacity or thermal capacity: C = Q/ΔT
SI Unit of Heat Capacity or thermal capacity: J K–1 or J °C–1
Definition & formula of Specific heat capacity
Specific heat capacity is the energy required to raise a unit mass of a substance by 1 K. ‘Specific’ here just means ‘per unit mass’.
The formula of Specific Heat Capacity: c = Q/(mΔT)
SI Unit of Specific Heat Capacity: J kg-1 K–1 or J kg-1 °C–1
specific heat capacity – Examples & values
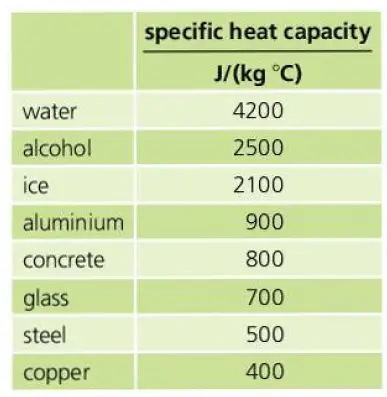
Water has a specific heat capacity of 4200 J/(kg °C). Aluminium has a specific heat capacity of only 900 J/(kg °C).
This means, 4200 joules of energy are needed to raise the temperature of 1 kg of water by 1 °C. Similarly, 900 joules of energy are needed to raise the temperature of 1 kg of Aluminium by 1 °C.
Here is an infographic showing the specific heat capacity of different materials.
Explanation of different Heat capacity & Specific heat capacity of different materials
In theory, if an object could be heated up with no energy loss, then the increase in temperature ΔT depends on three things:
• the energy that is given to the object Q,
• the mass, m, and,
• the substance from which the object is made.
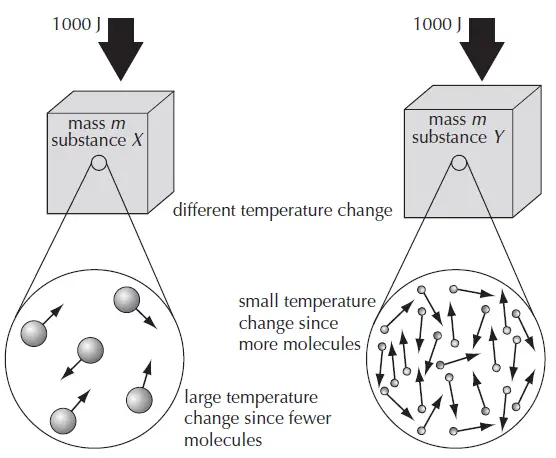
Two different blocks with the same mass and same energy input will have a different temperature change.
In the following diagram, two different blocks of the same mass but made of different substances (say X and Y) are supplied with the same energy (say 1000 J). It is observed that the two blocks undergo different temperature changes. Substance X with fewer molecules will have a larger temperature change compared to substance Y with more molecules. This happens due to different heat capacities and specific heat capacities of X and Y substances.
Methods of measuring Specific heat capacity
Let’s study a few methods of measuring Specific heat capacity. There are two basic ways to measure heat capacity.
Measuring specific heat capacity using the Electrical method
The experiment would be set up as below:
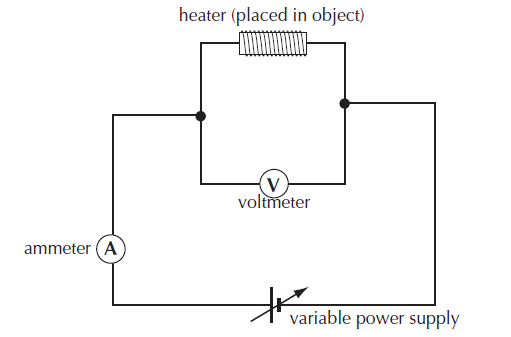
the specific heat capacity c= (I V t) / m (T2-T1) = ( current x voltage x time ) / (mass x change in temperature)
Sources of experimental error
• the loss of thermal energy from the apparatus.
• container for the substance and the heater will also be warmed up.
• it will take some time for the energy to be shared uniformly through the substance.
Measuring specific heat capacity using the Method of mixtures
The known specific heat capacity of one substance can be used to find the specific heat capacity of another substance.
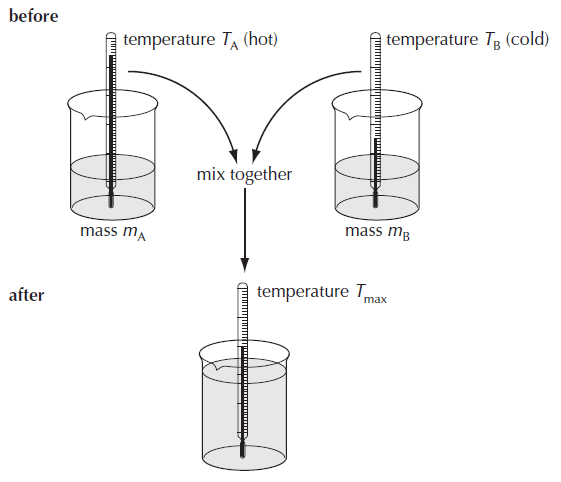
Procedure:
• measure the masses of the liquids mA and mB.
• measure the two starting temperatures TA and TB.
• mix the two liquids together.
• record the maximum temperature of the mixture Tmax.
If no energy is lost from the system then,
the energy lost by hot substance cooling down = energy gained by cold substance heating up
mA cA (TA – Tmax) = mB cB (Tmax – TB)
Now if we know the specific heat capacity of one substance in the equation, then the specific heat capacity of the other one in the equation can be calculated.
Again, the main source of experimental error is the loss of thermal energy from the apparatus – particularly while the liquids are being transferred. The changes in temperature of the container also need to be taken into consideration for a more accurate result.
Calculation of energy using the formulas
The energy that must be transferred to an object to increase its temperature can be calculated using this equation:
energy transferred = mass X specific heat capacity X temperature change
In symbols: energy transferred = m c (T2 – T1)
where m is the mass in kg, c is the specific heat capacity in J/(kg °C), and (T2-T1) represents the temperature change in °C (or in K).
The same equation can also be used to calculate the energy transferred when a hot object cools down.
