Latent heat and specific latent heat – definitions & concepts
In this post, we will discuss the fundamentals of latent heat and specific latent heat.
- latent heat – definition
- fusion | vaporization
- What happens to the thermal energy during phase change
- Do molecules speed up during a phase change?
- Why does the temperature stop rising at 0°C and 100°C, if heat energy is fed into a block of ice in a steady and even way?
- specific latent heat – definition
- Latent – meaning | summary | Takeaway
latent heat – definition
Latent heat is the amount of energy released or absorbed by a substance during a change of state that occurs without a change in temperature.
The amount of energy associated with the phase change is called the Latent heat.
fusion | vaporization
The technical term for the change of phase from solid to liquid is fusion. And the technical term for the change from liquid to gas is vaporization.
Melting and boiling are, of course, examples of changes of state, from the solid-state to the liquid state or from liquid to gas.
Whenever a substance changes state, latent heat is involved.
What happens to the thermal energy during phase change
- When a substance changes phase, the temperature remains constant even though thermal energy is still being transferred.
- The energy given to the molecules does not increase their kinetic energy so it must be increasing their potential energy.
- Intermolecular bonds are being broken and this takes energy.
- When the substance freezes bonds are created and this process releases energy.
Do molecules speed up during a phase change?
It is a very common mistake to think that the molecules must speed up during a phase change. The molecules in water vapour at 100 °C must be moving with the same average speed as the molecules in liquid water at 100 °C.
Why does the temperature stop rising at 0°C and 100°C, if heat energy is fed into a block of ice in a steady and even way?
Figure 1 shows what should happen if heat energy is fed into a block of ice in a steady and even way. Despite this constant input of
heat energy the temperature twice stays fixed: once at 0°C as it melts and once at 100°C as it boils. The extra energy you are supplying at these 2 temperatures does not show itself as a rise in temperature, and it is given the name latent heat. ‘Latent’ means hidden.
At 0°C as the ice melts, the heat energy it is gaining makes it no hotter, but instead, it goes into breaking down the rigid structure of the solid.
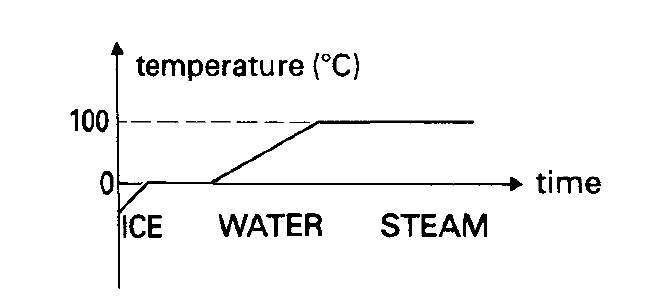
At 100°C the energy goes not into making the molecules move faster, but instead, it is used to separate the molecules from each other. Work has to be done in moving them apart against the attractive forces which hold them together as a liquid.
specific latent heat – definition
The specific latent heat of a substance is defined as the amount of energy per unit mass absorbed or released during a change of phase.
Also read How to measure specific latent heat – 2 setups and formulas
Latent – meaning | summary | Takeaway
If you heat a body, it will get hotter. That seems pretty obvious, so it may come as a surprise to discover that it is not necessarily true.
If you imagine being given a beaker containing water at 100°C and a Bunsen burner, you will begin to see why. You can continue to feed thermal energy into that water, but a thermometer would persist in reading 100°C. The extra energy you are supplying does not show itself as a rise in temperature, and it is given the name latent heat. ‘Latent’ means hidden.
The water of course is boiling away and turning to steam. The energy goes not into making the molecules move faster, but instead, it is used to separate the molecules from each other. Work has to be done in moving them apart against the attractive forces which hold them together as a liquid.
Something similar happens as ice melts. All that time the heat energy it is gaining makes it no hotter, but instead, it goes into breaking down the rigid structure of the solid.

