Specific latent heat of fusion – REVISION NOTES
The specific latent heat of fusion (lf) of a substance is the quantity of heat needed to change the unit mass of it from solid to liquid without temperature change.
role of latent heat of fusion
The kinetic theory explains latent heat of fusion as being the energy that enables the molecules of a solid to overcome the intermolecular forces that hold them in place, and when it exceeds a certain value they break free. Their vibratory motion about fixed positions changes to the slightly greater range of movement they have as liquid molecules, and the solid melts.
The energy input is used to increase the potential energy (p.e.) of the molecules, but not their average kinetic energy (k.e.) as happens when the heat causes a temperature rise.
temperature remains constant during melting – how?
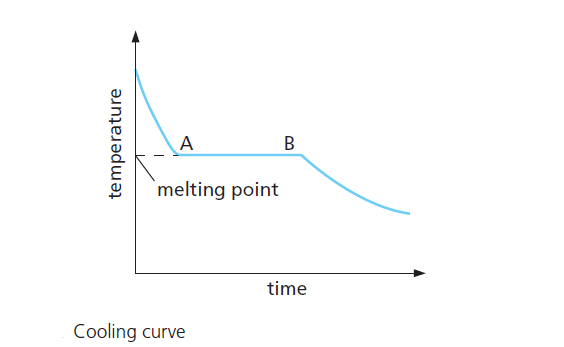
The cooling curve above shows that the temperature of the liquid ( pure substance ) falls until it starts to solidify and remains constant until it has all solidified.
The cooling curve in Figure 1 is for a pure substance; the flat part AB occurs at the melting point when the substance is solidifying.
During AB, the temperature remains constant.
During solidification, a substance loses heat to its surroundings but its temperature does not fall.
Conversely, when a solid is melting, the heat supplied does not cause a temperature rise; heat is added but the substance does not get hotter.
For example, the temperature of a well-stirred ice–water mixture remains at 0 ºC until all the ice is melted.
The heat that is absorbed by a solid during melting or given out by a liquid during solidification is called the latent heat of fusion. ‘Latent’ means hidden and ‘fusion’ means melting. Latent heat does not cause a temperature change; it seems to disappear.
unit
Unit of Specific latent heat: Specific latent heat is measured in J/kg or J/g.
formula
In general, the quantity of heat Q to change a mass m from solid to liquid is given by Q = m x lf = mass x specific latent heat of fusion
Hence, specific latent heat of fusion = Heat (Q) / mass (m)
