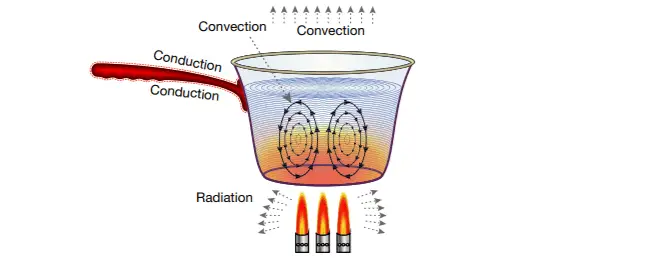
Conduction, Convection, & Radiation – Energy transfer during heating & cooling
Last updated on October 2nd, 2023 at 04:23 pm
There are three different processes through which energy can be transferred during heating and cooling: conduction, convection, and radiation. Conduction is the transfer of heat through a substance as a result of collisions between neighboring vibrating particles. Convection is the transfer of heat through a substance as a result of the movement of particles between regions of different temperatures. Heat can be transferred without the presence of particles by the process of radiation.
Conduction
Conduction is the transfer of heat through a substance as a result of collisions between neighboring vibrating particles. The particles in the higher temperature region have more random kinetic energy than those in the lower temperature region. As shown in Figure 2, the more energetic particles collide with the less energetic particles, giving up some of their kinetic energy. This transfer of kinetic energy from particle to particle continues until thermal equilibrium is reached. There is no net movement of particles during the process of conduction.
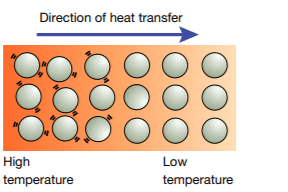
Solids are better conductors of heat than liquids and gases. In solids, the particles are more tightly bound and closer together than in liquids and gases. Thus, kinetic energy can be transferred more quickly. Metals are the best conductors of heat because free electrons are able to transfer kinetic energy more readily to other electrons and atoms.
Materials that are poor conductors are called insulators. Materials such as polystyrene foam, wool, and fiberglass batts are effective insulators because they contain pockets of still air. Air is a very poor conductor of heat. If air is free to move, however, heat can be transferred by a different method — convection.
Convection
Convection is the transfer of heat through a substance as a result of the movement of particles between regions of different temperatures. Convection takes place in liquids and gases where particles are free to move around. In solids, the particles vibrate about a fixed position and convection does not generally occur, except under specific conditions (e.g. in the extreme temperature and pressure in the Earth’s mantle).
The movement of particles during convection is called a convection current. Faster-moving particles in hot regions rise while slower-moving particles in cool regions fall. The particles in the warm water near the flame in Figure 3 are moving faster and are further apart than those in the cooler water further from the flame. The cooler, denser water sinks, forcing the warm, less dense water upwards. This process continues as the warm water rises, gradually cools, and eventually sinks again, replacing newly heated water.
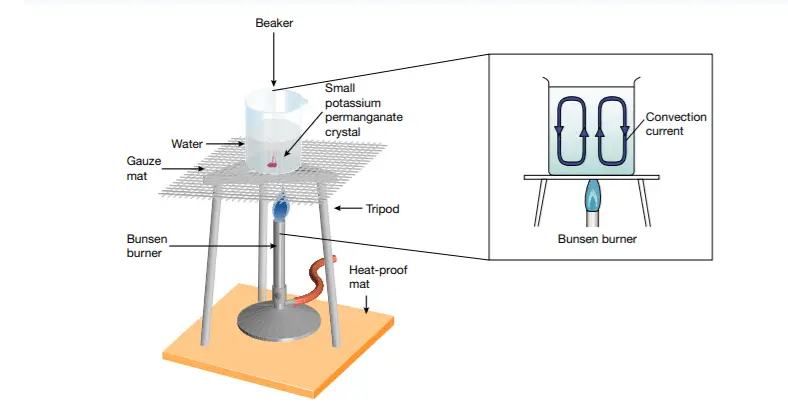
beaker are forced around the beaker by convection currents in the heated water
Convection currents are apparent in ovens that do not have fans. As the air circulates, the whole oven becomes hot. However, the top part of the oven always contains the hottest, least dense air.
As the air cools, it sinks and is replaced by less dense hot air for as long as the energy source at the bottom of the oven remains on. Fans can be used to push air around the oven, providing a more even temperature.
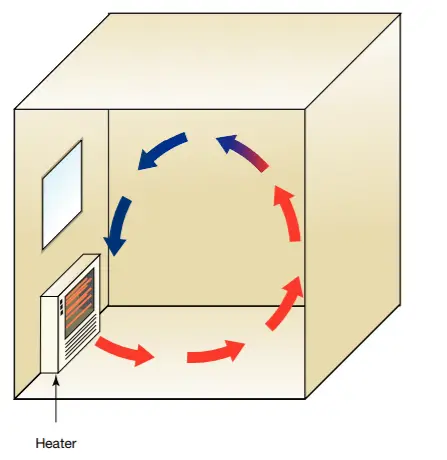
Home heating systems use convection to move warm air around. Ducted heating vents are, where possible, located on the floor. Without the aid of powerful fans, the warm air rises, and circulates around the room until it cools and sinks, being replaced with more warm air.
In homes built on concrete slabs, ducted heating vents are in the ceiling. Fans are necessary to push the warm air downwards so that it can circulate more efficiently.
In summer, loose-fitting clothing is more comfortable because it allows air to circulate.
Thus, heat can be transferred from your body by convection as the warm air near your skin rises and escapes upwards.
Radiation
Heat can be transferred without the presence of particles by the process of radiation. All objects with a temperature above absolute zero (0 K) emit small amounts of electromagnetic radiation. Visible light, microwaves, infrared radiation, ultraviolet radiation, and X-rays are all examples of electromagnetic radiation. All electromagnetic radiation is transmitted through empty space at a speed of
3.0 × 10^8 m/s, which is most commonly known as the speed of light.
Electromagnetic radiation can be absorbed by, reflected from, or transmitted through substances.
Scientists have used a wave model to explain much of the behavior of electromagnetic waves.
These electromagnetic waves transfer energy and reflect and refract in ways that are similar to waves on water.
What distinguishes the different types of electromagnetic radiation from each other is:
• their wavelength (the distance the wave takes to repeat itself)
• their frequency (the number of wavelengths passing every second)
• the amount of energy they transfer
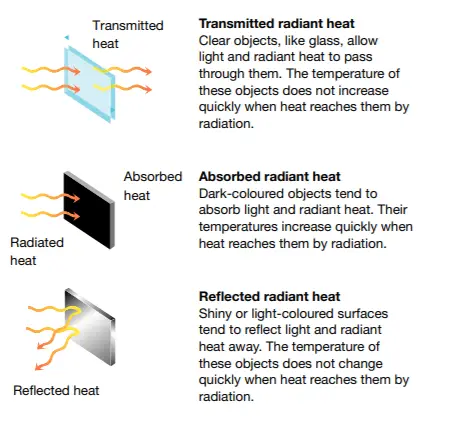
absorbed or reflected
These properties, in turn, determine their ability to be transmitted through transparent or opaque objects, their heating effect, and their effect on living tissue.
Figure 6 shows the electromagnetic spectrum and demonstrates that higher energy radiation corresponds to low wavelength.
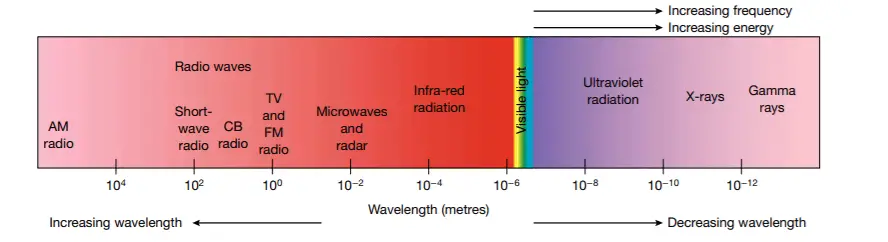
figure 6 The electromagnetic spectrum. All objects emit some electromagnetic radiation.
Why do hot objects emit electromagnetic radiation?
All matter is made up of atoms. At any temperature above absolute zero, these atoms are moving and colliding with each other. The atoms contain positive and negative charges. The motion of the atoms and their collisions with other atoms affect the motion of the electrons.
Because they are charged and moving around, the electrons produce electromagnetic radiation. Electrons moving in an antenna produce a radio signal, but in a hot object, the motion is more random with a range of speeds.
So, a hot object produces radiation across a broad range of wavelengths. If its temperature increases, the atoms move faster and have more frequent and more energetic collisions. These produce more intense radiation with higher frequencies and shorter wavelengths.
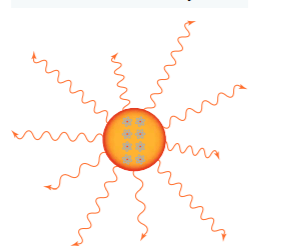
radiation from a hot body
Note: Numerical problems: Here is the link to one useful post in this portal where you will find a good collection of solved numerical problems on energy conversion or transformation.
