Activation energy & chemical Reaction – chemistry Study notes
Activation energy is the minimum energy required to start a chemical reaction. The colliding particles must possess at least this amount of energy for a reaction to take place.
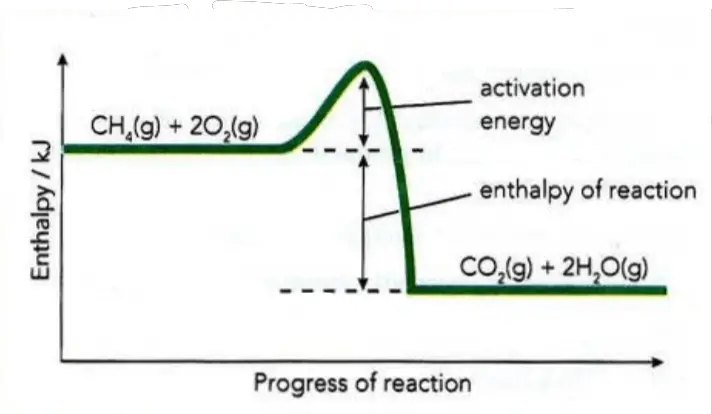
Most of the reactions are exothermic but only a few are totally spontaneous and begin without help at normal temperatures (e.g. sodium or potassium reacting with water). More usually, additional energy is required to start the reaction. When fuels are burnt, for example, energy is needed to ignite them (Figure 1). This energy may come from a spark, a match, or sunlight. It is called activation energy (Ea).
Activation energy – why is it required for a reaction to happen?
Activation energy is required because initially some bonds must be broken before any reaction can take place. Sufficient atoms or fragments of molecules must be freed for the new bonds to begin forming. Once a chemical reaction is started & energy is released with the formation of new bonds, the reaction continues.
For a chemical reaction to happen, some bonds in the reactants must first break before any new bonds can be formed. That is why all reactions require some activation energy.
For the reaction of sodium or potassium with water, the activation energy is low, and there is enough energy available from the surroundings at room temperature for the reaction to begin spontaneously.
Other exothermic reactions have higher activation energy (e.g. the burning of magnesium can be started with heat from a Bunsen burner). Reactions can be thought of as the result of collisions between atoms, molecules, or ions.
In many of these collisions, the colliding particles do not have enough energy to react and they just bounce apart. A chemical reaction will only happen if the total energy of the colliding particles is greater than the required activation energy of the reaction.
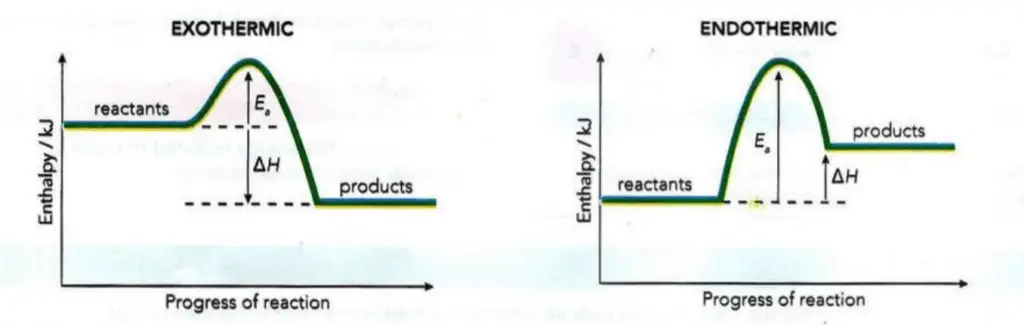
Activation energy ( Ea ) and the Enthalpy of reaction (ΔH)
A consideration of activation energy ( Ea ) and the enthalpy of reaction (ΔH) means that we can develop the idea of a reaction pathway diagram.
Figure 2 shows how the values of ΔH and Ea, can be represented on the reaction pathway diagrams for an exothermic and an
endothermic reaction. The data for a particular reaction will determine which profile to use.