Acid, Alkali, and pH scale – chemistry notes
Last updated on February 28th, 2022 at 11:19 am
Here you will get the fundamental concepts of acids, alkalies, and pH value (concepts, measurements, & examples).
What is an Acid?
An acid is a substance that produces hydrogen ions, H+, in an aqueous solution. For example, solutions of:
● hydrochloric acid (HCl) contains hydrogen (H+) ions and chloride (Cl–) ions
● sulfuric acid (H2SO4) contains hydrogen (H+) ions and sulfate (SO42–) ions
● nitric acid (HNO3) contains hydrogen (H+) ions and nitrate (NO3–) ions.
What is an Alkali?
An alkali is a substance that produces hydroxide (OH–) ions, in an aqueous solution. For example, solutions of:
● sodium hydroxide (NaOH) contain sodium (Na+) ions and hydroxide (OH–) ions
● potassium hydroxide (KOH) contain potassium (K+) ions and hydroxide (OH–) ions
● calcium hydroxide (Ca(OH)2) contain calcium (Ca2+) ions and hydroxide (OH–) ions.
The pH scale
The pH scale is a measure of how acidic or alkaline a solution is.
A solution with a pH of 7 is neutral, whereas a solution with a pH below 7 is acidic and one with a pH above 7 is alkaline.
The further away from 7 the pH is, the more acidic or alkaline the solution is (Figure 1).
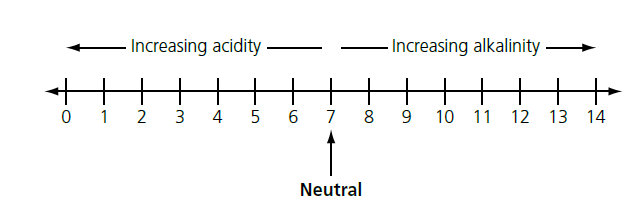
The scale is often shown as running from 0 to 14, but it does go further in both directions.
For example, it is common for the solutions of acids in school laboratories to have a pH that is less than 0 (typically about −0.3).
pH scale – value ranges
Chemists use a scale of numbers from about zero to 14, called the pH scale. The pH of a solution gives a measure of its acidity or alkalinity.
On this pH scale:
acidic solutions have a pH below 7
alkaline solutions have a pH above 7
neutral solutions have a pH of 7.
testing for acids using indicators | litmus test
The most convenient method of testing for acids is to use indicators, like litmus and universal indicators.
Indicators are substances that change color depending on how acidic or how alkaline a solution is.
Acidic solutions turn litmus red.
Acidic solutions give an orange or red color with a universal indicator.
Alkaline solutions turn litmus blue.
Alkaline solutions give a green, blue, or violet color with a universal indicator.
Measuring pH using universal indicator solution
The approximate pH of a solution can be measured using a universal indicator solution. A few drops of the indicator are added to the solution. The color is compared to a color chart to give the approximate pH of the solution (Figure 2).
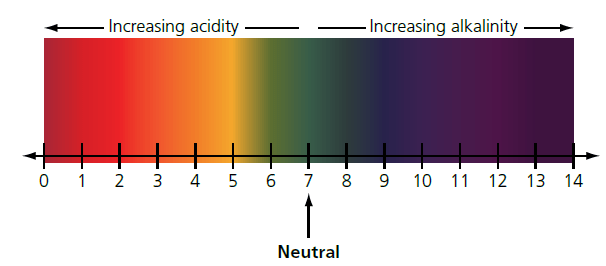
Using a universal indicator, it is possible to measure the approximate pH values of solutions. From these pH values, solutions can be classified as strongly acidic, weakly acidic, neutral, weakly alkaline, or strongly alkaline (Figure 2).
For example, battery acid (containing sulfuric acid) is strongly acidic, lemon juice containing ethanoic (acetic) acid is weakly acidic, water is neutral, and drain cleaner containing sodium hydroxide is strongly alkaline.
Measuring pH using pH probe
A more accurate way of finding the pH of a solution is to use a pH probe (Figure 3). There are different types but the probe is dipped into the solution and the pH shown on the display, often to 1 or 2 decimal places.

pH is based on the concentration of H+ ions in the solution
The pH of a solution is based on the concentration of H+ ions in the solution.
The higher the concentration of H+ ions the lower the pH.
As the pH decreases by one unit, the concentration of hydrogen ions increases by a factor of 10.
For example, a solution with a pH of 2 has a concentration of H+ ions that is 10 times greater than one with a pH of 3.
A solution with a pH of 1 has a concentration of H+ ions that is 100 times greater than one with a pH of 3.
In a neutral solution, the concentration of H+ ions equals the concentration of OH– ions.

