Enthalpy – chemistry revision notes for class 11
Last updated on January 16th, 2022 at 10:59 am
Enthalpy of a system may be defined as the sum of the internal energy(U) and the product of its pressure(p) and volume(v).
It is denoted by the symbol H and is given by:
H = U + pV
Getting the Heat Transfer equation (derivation)
Change in the internal energy of a system is:
ΔU= q + w = q – PΔV
where q has a positive sign if the system gains heat and a negative sign if the system loses heat.
Rearranging the equation to solve for q gives the amount of heat transfer:
q= ΔU+ PΔV ……………. (1)
Let’s look at two ways in which a chemical reaction might be carried out.
change in internal energy is equal to the heat absorbed or evolved at constant volume & constant temperature
A) A reaction might be carried out in a closed container with a constant volume so that ΔV = 0.
In such a case, no PV work can be done so the energy change in the system is due entirely to heat transfer. We indicate this heat transfer at constant volume by the symbol qv.
So from equation (1) above, we get
qv = ΔU [At constant volume; ΔV = 0] ……………….(2)
The change in internal energy is equal to the heat absorbed or evolved at constant volume and constant temperature.
heat of reaction, or enthalpy change (𝚫H) | Enthalpy equation
B) Alternatively, a reaction might be carried out in an open flask or other apparatus that keeps the pressure constant and allows the volume of the system to change freely.
In such a case, ΔV is non-zero and the energy change in the system is due to both heat transfer and PV work.
We indicate the heat transfer at constant pressure by the symbol qp:
qp = ΔU + PΔV [At constant pressure] …………. (3)
Because reactions carried out at constant pressure in open containers are so common in chemistry, the heat change qp for such a process at constant pressure is given a special symbol and is called the heat of reaction, or enthalpy change (𝚫H).
ΔH= ΔU + PΔV [At constant pressure] ……………… (4)
Definition: the heat change qp at constant pressure is given a special symbol and is called the heat of reaction, or enthalpy change (𝚫H)
The enthalpy (H) of a system is the name given to the quantity U + PV.
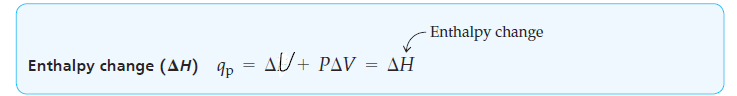
[ Also Read: Thermodynamics Notes (Chemistry)]
Relationship between ΔH and ΔU
The relationship between ΔH and ΔU can be presented by the following equations.
ΔH = ΔU + pΔV…………..(i)
where ΔU is the change in internal energy and ΔV is the change in volume of the system
This relationship between ΔH and ΔU can be presented by the following equation as well:
qp = qv + PΔV ……………… (ii)
enthalpy change is a measure of heat change (evolved or absorbed) taking place during a process at constant pressure
Now, enthalpy is defined as
H = U + pV ……….(1)
Change in enthalpy, ΔH may be expressed as
ΔH = ΔU + Δ(pV)
ΔH = ΔU + pΔV + VΔp
If the process is carried out at constant pressure,
i.e., Δp = 0, then
ΔH = ΔU + pΔV …….. (2)
Now, ΔU = q + w
If work done during the change is only expansion work,
w = −pΔV so that
ΔU = q − pΔV ……..(3)
ΔH = ΔU + pΔV = q − pΔV + pΔV = q
∴ ΔH = qp (at constant pressure)
where qp indicates that heat change has taken place at constant pressure.
Thus, enthalpy change is a measure of heat change (evolved or absorbed) taking place during a process at constant pressure.
Enthalpy – important points
- If q is the amount of heat absorbed by the system then:
q (at constant volume) = ΔU
q (at constant pressure) = ΔH
- Note that only the enthalpy change during a reaction is important.
- As with internal energy, enthalpy is a state function whose value depends only on the current state of the system, not on the path taken to arrive at that state. Thus, we don’t need to know the exact value of the system’s enthalpy before and after a reaction. We need to know only the difference between final and initial states:
- ΔH = Hfinal – Hinitial = Hproducts – Hreactants
- Enthalpy is also called heat content. Since H depends upon three state functions U, p, and V, it is also a state function.
[ Also Read: Thermodynamics Notes (Chemistry)]
