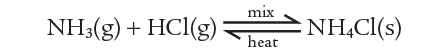Some reversible chemical reactions with equations
Reactions that can be reversed are called reversible reactions.
Most of the chemical reactions are irreversible. But there are some processes that can be reversed.
ice to water, and water to ice
For example, ice turns into the water on heating:
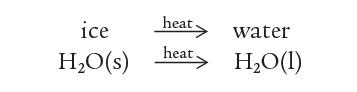
If the water is now cooled, ice re-forms.
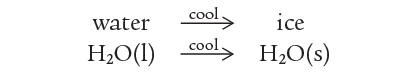
These two parts of this reversible process (not a reaction) can be combined in one equation as:
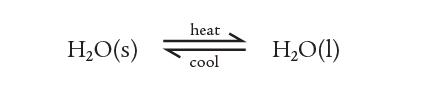
Two more reversible reactions with equations
1)
When blue hydrated copper sulfate is heated, it decomposes to white anhydrous copper sulfate and water vapor.
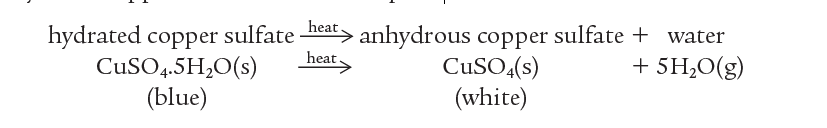
If water is now added, the change can be reversed and blue hydrated copper sulfate re-forms.
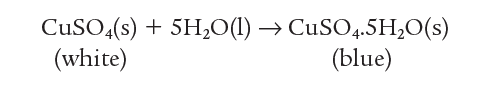
These two processes can be combined in one equation as:
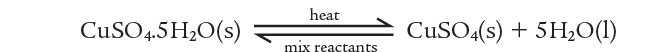
2)
Another reversible reaction is that involving ammonia, hydrogen chloride, and ammonium chloride. Ammonia and hydrogen chloride react at room temperature to form a white smoke which is a suspension of solid ammonium chloride:
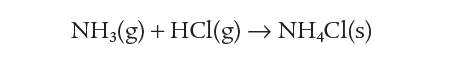
If ammonium chloride is heated, this reaction is reversed. The ammonium chloride decomposes to ammonia and hydrogen chloride. So the reversible reaction can be summarised as: