Alkanes & The first four alkanes – revision notes
Alkanes are a family of saturated hydrocarbons. Saturated molecules are ones that only contain single covalent bonds. The structures of the first four alkanes are shown in table 1. The table includes the displayed formula which shows all the atoms and all the bonds in each molecule.
The first four alkanes
The first four alkanes are Methane, Ethane, Propane, and Butane.
The following table shows the Ball and stick structure, displayed formula, and molecular formula of the first four alkanes.
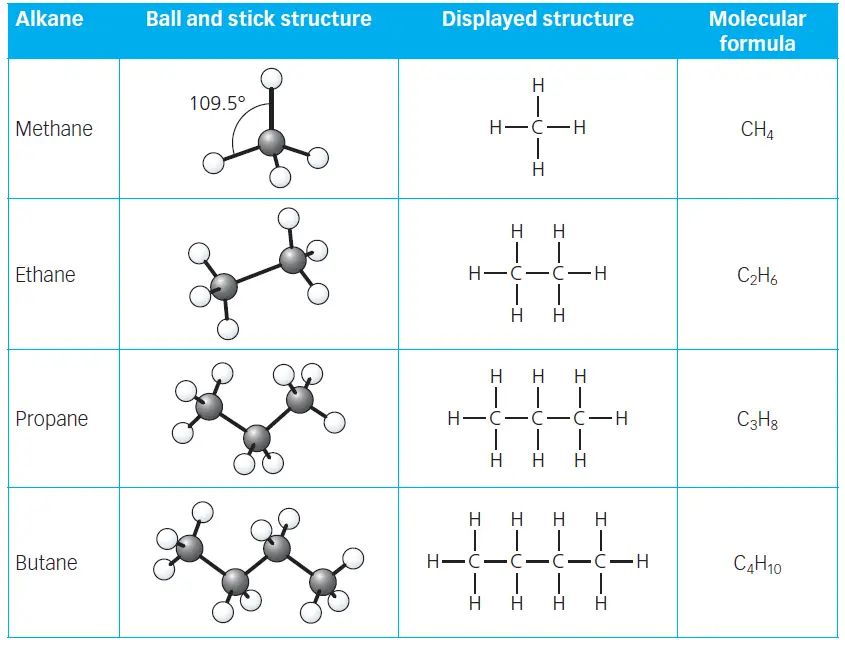
All the alkanes have a molecular formula of the form CnH2n+2. For example, if there are 3 carbon atoms (n = 3), then there are 8 hydrogen atoms (2n + 2 = (2 × 3) + 2 = 8). This and other examples are shown in Table 2.

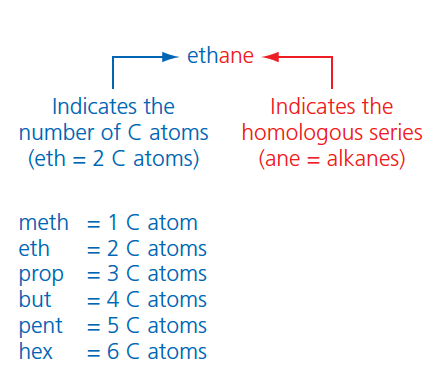
The names of organic compounds are made up of two parts. The first part of the name indicates the number of carbon atoms and the second part indicates which homologous series the molecule belongs to (Figure 3).
The alkanes are an example of a homologous series. A homologous series is a family of compounds with:
● the same general formula
● similar chemical properties.
