Surface Tension with molecular theory – class 11 physics
Last updated on March 11th, 2022 at 03:36 pm
The surface of a liquid at rest behaves like a stretched membrane. It tries to contract and have a minimum surface area. This property of the liquid surface is known as surface tension.
Illustrations of Surface Tension | examples of surface tension
- Small liquid drops are always spherical in shape. It can be shown that for a given volume; the surface area of a sphere is minimum. The spherical shape of drop indicates that liquid acquires a shape so that its surface area is minimum.
- A large mercury drop is broken into small droplets on the base of a clean dish. The small droplets formed have sherical shape. This is due to surface tension.
- Place a greased iron needle on a blotting paper and gently place it over surface of water in a beaker. Very soon blotting paper sinks inside water but iron needle remains floating on surface of water! Careful observations of surface of water with a microscope shows a small depression where of needle is. The water surface behaves like a streteched membrane. The weight of needle is balanced by the vertical component of forces of surface tension.
Molecular Theory of Surface Tension | free-energy of the liquid surface
In figure 1, XX’ is the surface of a liquid at rest. X1 X11 is at a distance equal to the range of interatomic /molecular forces. The liquid between XX’ and X1 X11 is known as surface film.
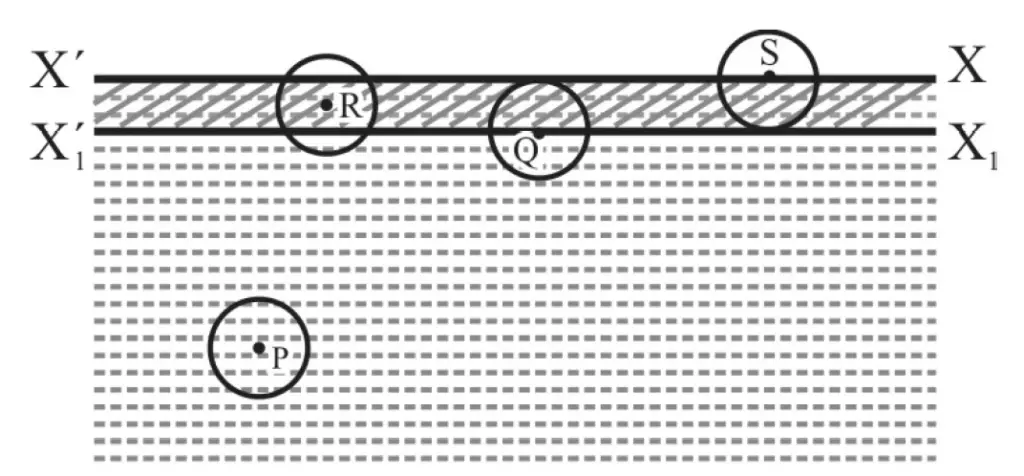
For atom or molecule P; the “sphere of influence” (i.e. sphere of radius equal to the range of interatomic/molecular force with P as center) is completely within the liquid. Net interatomic/molecular force on P is zero.
The same is true for atom/molecule Q lying on the lower face of the surface film. No work is done by or against interatomic/molecular force when P moves up to Q.
For atom / molecular at R; there is a net downward interatomic/molecular force and it is maximum for atom/molecule at S (i.e. on surface film).
When an atom or molecular moves from Q to S, it has to do work against interatomic/molecular force.
In other words, potential energy due to interatomic/molecular forces of an atom/molecule on surface XX’ is more than that of an atom/molecule at X1 X11 or below.
This excess potential energy per unit area is known as the free–energy of the liquid surface.
Later we will see that free energy of liquid surface per unit area = surface tension
Related Notes
- Dimensional formula of surface energy per unit area
- Surface tension equals the free energy of the liquid surface
- Difference between cohesion and adhesion
- Dimensional formula of surface tension
- Excess pressure across a curved liquid surface & surface tension
- Molecular theory of solid, liquid, and gas – for their shape & volume
Take Away
We know that the equilibrium state of any system is one of minimum potential energy. For the liquid surface, this will be so if the liquid surface tries to acquire minimum surface area. This is the surface tension effect.
