Carbon & its bonding, allotropes, Physical Nature & Compounds
Carbon is non-metal. In nature, it occurs in its pure form as diamond and graphite. When fuels burn, the carbon in them reacts with oxygen to form carbon dioxide.
Carbon compounds hold the key to plant and animal life on the earth. Carbon circulates through air, plants, animals, and soil by means of complex reactions. This is called the carbon cycle.
All living organisms are made of carbon atoms. This means that carbon atoms form the building blocks of living organisms. These carbon atoms, in combination with other atoms, decide life on earth. Hence carbon chemistry is also called living chemistry.
Bonding in Carbon and its Compounds
The atomic number of carbon is 6. Carbon’s ground state electronic configuration is 1s2 2s2 2p2. Since it has four electrons in its outermost shell, its valency is four.
To achieve noble gas configuration, a carbon atom has to lose or gain four electrons to form C4+ and C4- ions.
- It could gain four electrons forming C4- anions, but it would be difficult for the nucleus with six protons to hold on to ten electrons i.e. four extra electrons.
- It could lose four electrons to form C4+ cations, but it would require a large amount of energy to remove four electrons leaving behind the carbon cations with six protons in its nucleus holding on to just two electrons.
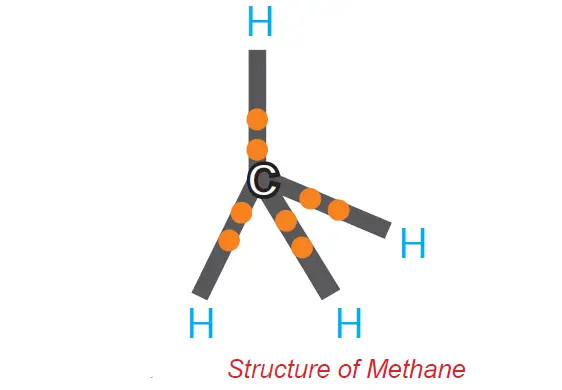
Carbon overcomes this problem by sharing its valence electrons with other atoms of carbon or with atoms of other elements. This characteristic of a carbon atom by virtue of which it forms four covalent bonds is generally referred to as tetra valency of carbon.
A molecule of methane (CH4) is formed when four electrons of carbon are shared with four hydrogen atoms.
Allotropy
Allotropy is defined as the property by which an element can exist in more than one form and those forms are physically different but chemically similar.
Allotropes of Carbon
Carbon exists in three allotropic forms. They are crystalline form (diamond and graphite), amorphous form (coke, charcoal), and fullerene.
diamond and graphite – crystalline allotropic forms of Carbon
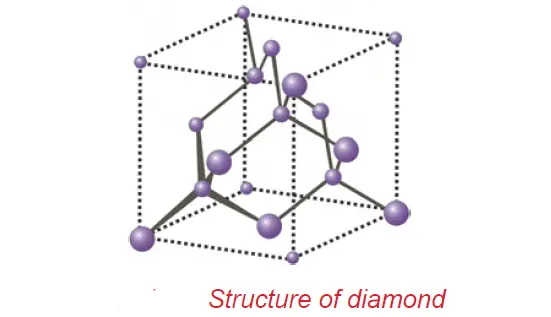
Diamond and graphite are crystalline allotropic forms. They differ in the nature of the bond.
• In diamond, each carbon atom is bonded to four other carbon atoms in a tetrahedral fashion leading to a rigid three-dimensional structure, accounting for its hardness and rigidity.
• In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving hexagonal layers held together by weak Van der Waals forces accounting for softness.
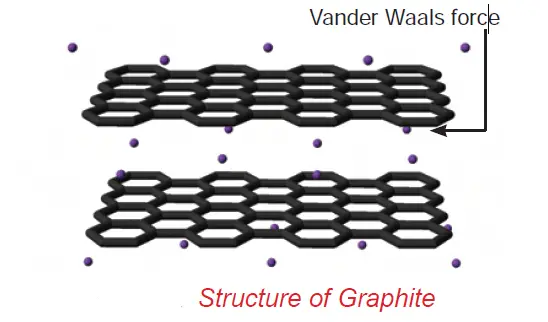
Graphite is a good conductor of electricity unlike other non-metals since it has free electrons in it.
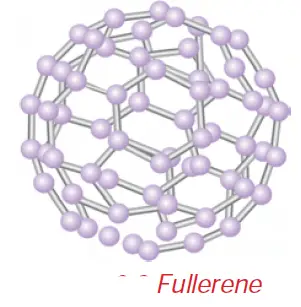
• Fullerenes form another type of carbon allotropes. The first one was identified to contain 60 carbon atoms in the shape of a football. (C-60). Since this looks like the geodesic dome designed by the US architect Buck Minster Fuller, it is named Buck Minster Fullerene.
Physical Nature of Carbon & its Compounds
- Carbon has the ability to form covalent bonds with other atoms of carbon giving rise to a large number of molecules through self linking property. This property is called catenation. Since the valency of carbon is four, it is capable of bonding with four other atoms.
- Carbon combines with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements to form various stable compounds.
- The stability of carbon compounds is due to the small size of carbon which enables the nucleus to hold on to the shared pair of electrons strongly.
- Carbon compounds show isomerism, the phenomenon by which two or more compounds have the same molecular formula but different structural formulas with differences in properties. That means, the formula C2H6O represents two different compounds namely ethyl alcohol (C2H5OH) and dimethyl ether (CH3OCH3).
- Carbon compounds have low melting and boiling points because of their covalent nature.
- The reactions shown by carbon compounds involve the breaking of old bonds in the reacting molecules and the formation of new bonds in the product molecules.
- Carbon compounds are easily combustible.
