How Bonding type decides Boiling and melting points
In this post, we will see how the type of bonding influences the melting point and the boiling point of a substance.
Simple covalent molecules such as H2, F2, O2, CH4, etc. are gases at room temperature. They form molecular crystals which have low melting and boiling points. The intermolecular interactions between these covalent molecules are only weak London dispersion forces. Therefore, they have low melting and boiling points. The polar molecules generally have higher boiling points than non-polar molecules of a similar mass because of the dipole-dipole forces of attraction between polar molecules.
It may be noted that in the case of ionic substances, exceedingly high temperatures are required to melt or boil these substances because of strong interionic forces. Similarly, the forces holding the atoms together in metals are very strong. Therefore, metals in general, have very high melting and boiling points.
three states of matter are interconvertible
Certainly, intermolecular forces and thermal energy have roles in determining a particular state of matter. These three states of matter are interconvertible by changing the conditions of temperature and pressure.
For example, water is a liquid at an ordinary temperature. It can be changed into steam (gas) by heating at 100°C whereas, it can also be changed into ice (solid ) by cooling below 0°C.
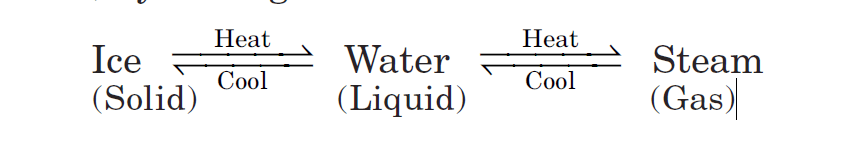
When a solid is heated, it melts to form a liquid.
The temperature at which solid melts to become a liquid is called its melting point.
On further heating, a liquid changes to vapor.
The temperature at which a liquid changes to vapor is known as its boiling point.
Intermolecular forces of attraction influence the temperature at which the substance melts or boils
The intermolecular forces of attraction between the particles constituting the matter (atoms, ions, or molecules) influence the temperature at which the substance melts or boils. If the intermolecular forces of attraction are strong, the melting point and boiling point will be high and if these forces are weak, the melting point and boiling point will be low.
For example, we expect to have low melting and boiling points of noble gases because only the weak London dispersion forces hold the atoms together in the solid or liquid states.
