Solubilities of various salts in water
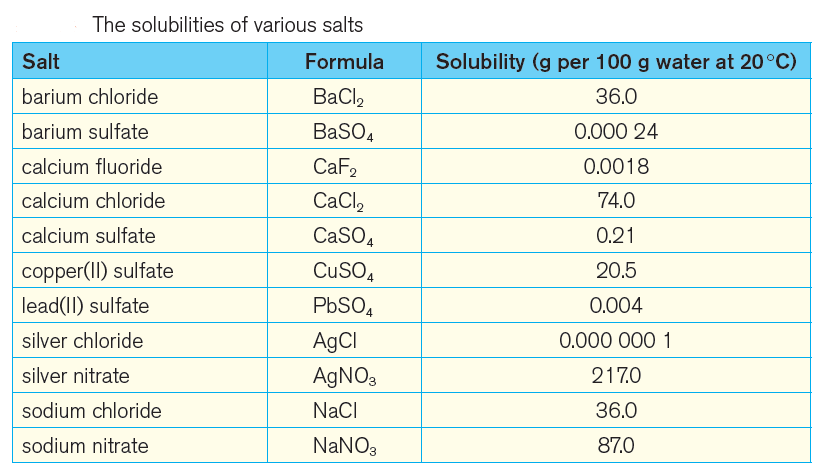
In this post, the solubilities of various salts in water at 20 °C are listed in a table (figure 1).
In this list, you can easily notice the wide range in solubilities, where silver nitrate has a very high solubility of 217 g per 100 g water and silver chloride has a very low solubility of 0.000 000 1 g per 100 g water.
Solubilities of various salts in water listed in an table (image)
soluble and insoluble salts – definition
It is useful to divide salts into two categories – soluble and insoluble.
Salts with a solubility greater than 1 g per 100 g water are classed as soluble; salts with a solubility less than 1 g per 100 g water are classed as insoluble.
The general rules for the solubilities of common salts
- All salts of sodium, potassium & ammonium are soluble.
- All nitrates are soluble.
- All sulphates are soluble except Ag2SO4, CaSO4, BaSO4, and PbSO4
- All chlorides are soluble except AgCl and PbCl2
- All carbonates are insoluble except those of Na+, K+, and NH4+.
