Naming convention of salts | The names of salts – rules
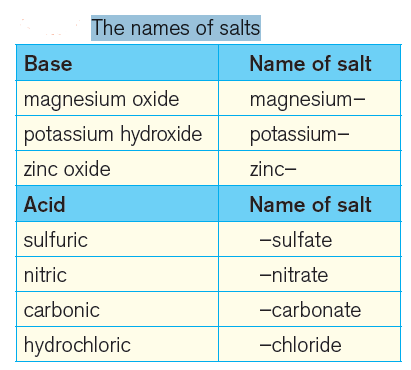
The data in the table above shows how the name of a salt comes from its parent base and acid. So, magnesium oxide and nitric acid would produce magnesium nitrate.
Acids with names ending in ‘-ic’ form salts with names ending in ‘-ate’.
Thus, the salt from sulphuric acid will have ‘sulphate’, salt from nitric acid will have nitrate in its name.
(Hydrochloric acid is an exception – its salts are called chlorides.)
