Redox (reduction-oxidation) reactions – revision notes
Last updated on January 24th, 2022 at 03:48 pm
Redox (reduction-oxidation) reactions involve electron transfer and include all chemical reactions in which atoms have changed their oxidation state.
Oxidation state is the state of an element or ion in a compound with regard to the electrons gained or lost by the element or ion in the reaction.
In many systems, the reactions occur in a region known as the cell, where the transfer of electrons occurs at electrodes.
redox reactions – definition
Oxidation is a loss of electrons (an increase in oxidation number) and reduction is a gain of electrons (a decrease in oxidation number).
redox reactions – real life examples
Dry cells or batteries use redox reactions to produce electrical energy that can be converted into useful work. Many metals may be purified or electroplated using electrochemical methods. Redox reactions also play a vital role in devices such as cars, smartphones, electronic tablets, watches, pacemakers, and many others uses, because these devices use batteries for power.
Redox reaction mechanism – explained
An example of a redox reaction involves the reaction between magnesium metal and oxygen to form magnesium oxide, as shown in Figure 1.
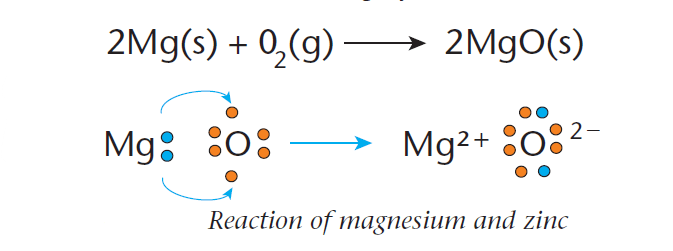
During this reaction, a magnesium atom (Mg) loses two electrons to form an Mg2+ ion.
Each oxygen atom (O2–) gains two electrons to form an oxygen ion.
Since the magnesium is losing electrons in the reaction, it is being oxidized. The oxygen is gaining electrons and is thus being reduced.
In general, redox reaction involves two species, for example, A and B as shown in Figure 2.
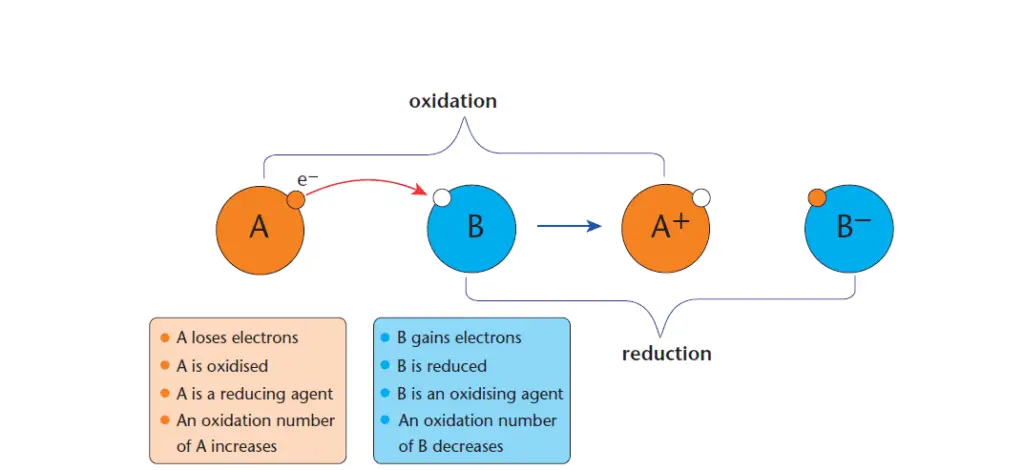
In this example, substance ‘A’ loses electrons and it is oxidized. A substance that is oxidized is a reducing agent and the process of losing electrons is called oxidation.
On the other hand, substance ‘B’ gains electrons and is thus being reduced. A substance that is reduced during a chemical reaction is called an oxidizing agent, and the process of gaining an electron is called a reduction.
