Thermodynamics Processes
Last updated on May 14th, 2022 at 02:15 pm
In this post, we will cover a few important & frequently asked questions on Thermodynamics Processes.
The state of a thermodynamic system can be changed by a process. In other words, a process gives the path or operation by which a system changes from one state to another. The process may be accompanied by an exchange of matter and energy between the system and the surroundings. This results in a change in at least one of the state variables of the system.
There are certain processes in which a particular state variable (thermodynamic property of the system) is kept constant. These are identified with special names.
Name different types of thermodynamic processes
- Reversible process
- Irreversible process
- Adiabatic process
- Isothermal process
- Isobaric process
- Isochoric process
- Cyclic process
Define different types of thermodynamic processes.
a) Reversible Process: A process that is carried out infinitesimally slowly by a series of steps such that the system and surroundings are always in near equilibrium with each other is called the Reversible Process. At any moment, this process can be reversed by an infinitesimal change.
b) Irreversible Process: A process that cannot be reversed by a small change is called the Irreversible Process. Irreversible processes are carried out at finite rates.
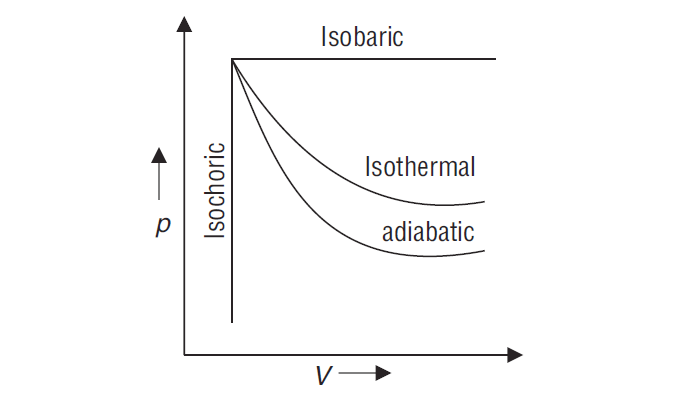
c) Adiabatic Process: A process during which no heat can flow in or out of the system is called the adiabatic process.
Here, dq = O
d) Isothermal Process: The process that is carried out at a constant temperature is called the Isothermal Process.
Here, dT = O
e) Isobaric Process: The process that is carried out at constant pressure is called the Isobaric Process.
Here, dp = O
f) Isochoric Process: The process that is carried out at constant volume is called the Isochoric Process.
In this case, dV = O
g) Cyclic Process: When a system undergoes a series of changes and finally returns to its initial state then the process is called the Cyclic process.
Draw p – V graph for various thermodynamic processes