electronvolt – what is electronvolt(eV) and how is eV related to Joule?
Last updated on April 12th, 2021 at 12:25 pm
The SI unit of energy joule is too large a unit of energy for the microscopic world. A more convenient unit of energy for the microscopic world is the electronvolt, eV. eV by the way is not part of the SI system.
What is electronvolt? | Define electronvolt
We define the electronvolt as the work done when a charge equal to one electron charge is taken across a potential difference of one volt.
When a charge equal to two electron charges is taken across a potential difference of 1 V, the work done is 2 eV; moving a charge equal to seven electron charges across a potential difference of 5 V results in work of 35 eV, and so on.
How to convert electronvolt to joule? | How electronvolt is related to Joule
Electronvolt is a convenient unit of energy and not an SI unit. The SI unit of energy is Joule. Hence, it’s a common interest to know the relationship between electronvolt and Joule.
To convert eV to Joule, we will use this formula: W = qV
An electronvolt is the work done to take a charge equal to one electron across a potential difference of one volt, so we will put the values of q and V accordingly in the equation.
1 eV = 1.6 × 10−19 C × 1 V = 1.6 × 10−19 J
So the relationship between electronvolt(eV) and Joule can be expressed by the equation: 1 eV = 1.6 × 10−19 J
Joule to electronvolt (eV) conversion
We know that 1 eV = 1.6 × 10−19 J. From this equation we get Joule to electronvolt (eV) conversion easily as shown below:
1 J = 1/(1.6 × 10−19 ) eV
Where is electronvolt used?
Electronvolt(eV) is a unit of energy that is commonly used in atomic and nuclear physics.
Can you convert electronvolt to Watt?
You can’t convert electronvolt to Watt, because electron-volt and Watt units represent different quantities. Electron-volt(eV) is the unit of energy and Watt is the unit of power.
Can you convert electronvolt to Volt?
You can’t convert electronvolt to Volt, because electron-volt and Volt units represent different quantities. Electron-volt(eV) is the unit of energy and Volt is the unit of potential difference.
Solving Numerical problems using eV
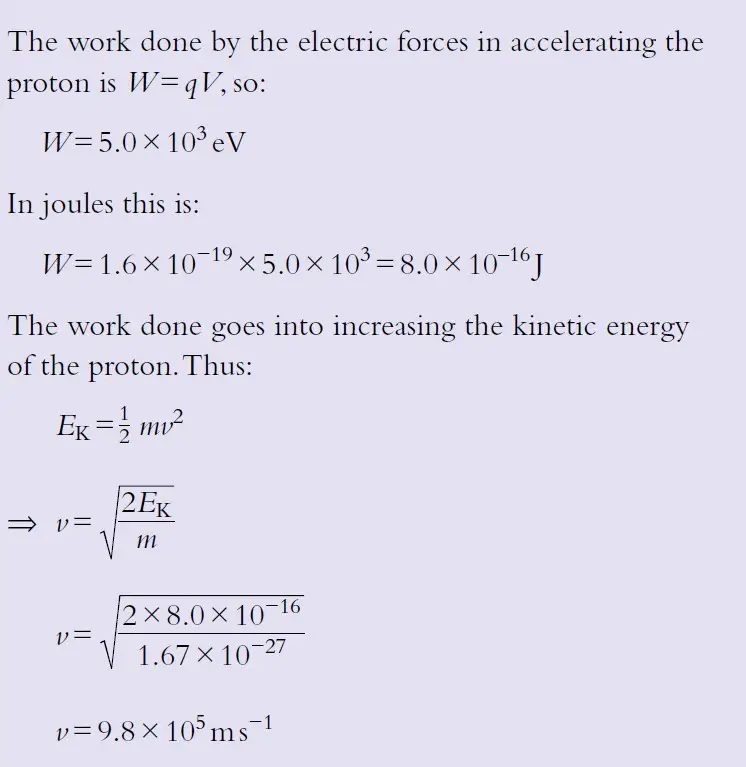
a) Determine the speed of a proton (m = 1.67 × 10–27 kg) that is accelerated from rest by a potential difference of 5.0 × 103 V.
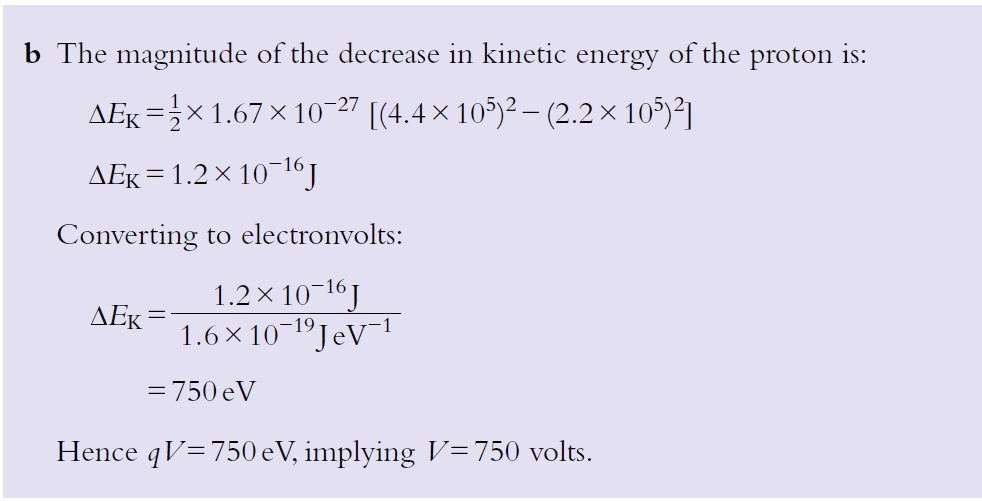
b) A proton with speed 4.4 × 106 m s−1 enters a region of electric field directed in such a way that the proton is slowed down.
Determine the potential difference required to slow the proton down to half its initial speed.
Solution:
(a)
Summary
We have seen how to define eV and how it is related to Joule. We have also gone through a number of frequently asked questions related to electronvolt. We have seen how to solve a numerical problem related to eV for better understanding.
Hope you have liked the post. Now please share this post using the share button on this page. Bookmark this site as well and visit other pages for interesting posts.
