Zeroth Law of Thermodynamics and thermal equilibrium
Last updated on December 30th, 2021 at 03:07 pm
This post is on Zeroth Law of Thermodynamics. To discuss this we will begin with a briefing on the definition of Thermodynamics and the concepts of Thermal Equilibrium in the context of Thermodynamics.
The Zeroth Law of Thermodynamics throws light on a major condition of thermal equilibrium among systems.
Thermodynamics – define it
Thermodynamics is the branch of physics that deals with the concepts of heat and temperature and the inter-conversion of heat and the other forms of energy.
What is the Equilibrium in Thermodynamics?
And Equilibrium in Thermodynamics is availed when the variables of the systems don’t change with time. For example, say a gas inside a closed rigid container is insulated from its surroundings. And as a result, say, its pressure, volume, temperature, mass, and composition remain unchanged. In this case, we can say that the gas has attained a state of equilibrium.
Zeroth law of Thermodynamics – statement
This states that if two systems are in thermal equilibrium with a third system separately then they (the first 2 systems) are in thermal equilibrium with each other as well.
Understanding Zeroth law of Thermodynamics with a 2 step process
Let’s discuss this in detail. We will a 2 step process to understand the basic concepts.
Step 1: Thermal Equilibrium in Thermodynamics
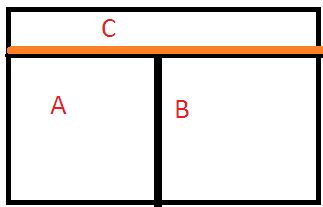
Let’s take 3 systems, A, B, and C.
System A and System B are isolated by an adiabatic wall (no conduction of heat), but each of these A and B, is individually isolated to a 3rd system C by a conducting wall.
As time passes, after some time A will come to thermal equilibrium with C and similarly, system B will have the same equilibrium state with C separately.
Now, understanding the fact that thermal equilibrium means temperature equality, we can write the following equations, as per the above law stated:
Ta = Tc …………….. (1) and
Tb = Tc …………………….(2)
[ Ta, Tb, and Tc are the temperature of the systems A, B, and C respectively.]
Step 2: Thermal Equilibrium in Thermodynamics
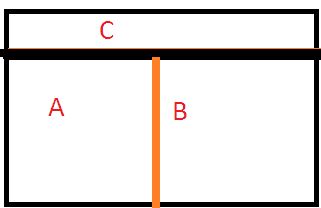
Now if we interchange the nature of the 2 walls, i.e. between A and B now the wall would be conducting and the other wall between C and the other 2 systems become adiabatic, then what is going to happen?
The wall separating C from others is adiabatic, there is no possible interaction with C. But there is ample scope of heat transfer now between A and B.
But, it is found that there is no further change of states for A and B. That means they are now in Thermal Equilibrium with each other.
That means as per the Zeroth Law of Thermodynamics, we can say, Ta = Tb.
zeroth law of Thermodynamics: Summing up
So summing up the above 2 steps we can write,
If Ta = Tc …………….. (1) and
Tb = Tc …………………….(2)
then Ta = Tb …………(3) [ at thermal equilibrium].
Equation (3) above is showcasing the zeroth law of Thermodynamics which states if two systems are in thermal equilibrium with a third system separately then the first 2 systems are in thermal equilibrium with each other as well.
Interesting Related Post:
Thermal Conductivity – definition, dimension, concepts, values

