How Kinetic theory of matter explains Changes of state?
The kinetic theory of matter can be used to explain how a substance changes from one state to another.
A summary of the different changes of state is shown in Figure 1.
These changes are usually caused by heating or cooling.
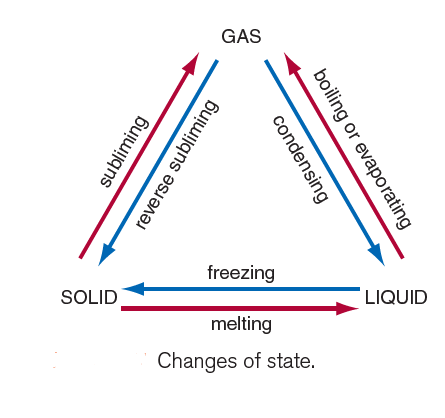
Explaining Melting and freezing with the help of the Kinetic theory of matter
When a solid is heated, its particles gain energy. The particles vibrate faster and faster until eventually, they break away from their fixed positions. The particles begin to move around each other and the solid has melted to form a liquid.
The temperature at which the solid melts is called the melting point.
The temperature at which a solid melts tells us how strongly its particles are held together.
Substances with high melting points have strong forces of attraction between their particles. Substances with low melting points have weak forces of attraction between their particles.
Metals and alloys, like iron and steel, have high melting points. This suggests that there are strong forces of attraction between their particles. This is why metals can be used as girders and supports.
How kinetic theory of matter explains Evaporating and boiling
The particles in a liquid can move around each other. Some particles near the surface of the liquid may have enough energy to escape from the liquid into the air. When this happens, the liquid evaporates to form a gas.
If the liquid is heated, its particles move faster. This gives more of them sufficient energy to escape from the surface. So, evaporation increases as the temperature of the liquid rises.
On further heating, the liquid particles are moving so rapidly that bubbles of gas form inside the liquid.
The temperature at which evaporation occurs in the bulk of the liquid is the boiling point.
Boiling points tell us how strongly the particles are held together in liquids.
Volatile liquids, like petrol, evaporate easily and boil at low temperatures. They have weak forces of attraction between their particles.
