The gaseous state of matter
Last updated on May 14th, 2022 at 02:19 pm
The gaseous state is the simplest state of matter. In this post, we will cover some important facts and frequently asked questions on gases and the gaseous state of matter.
Number of gas elements in the periodic table
A look at the periodic table shows that only eleven elements are gases under normal conditions as shown in the figure below.
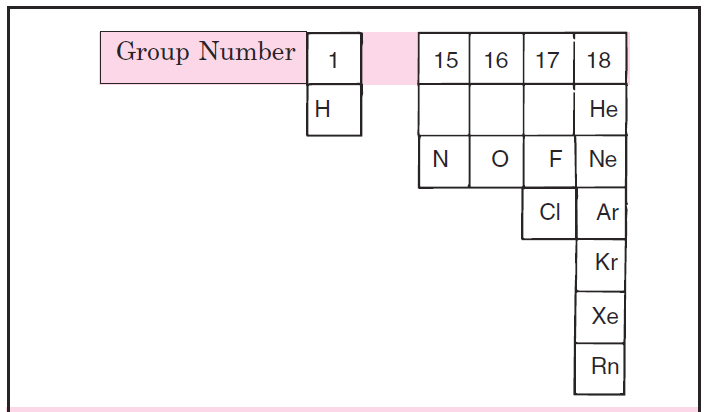
General Characteristics of Gases
The gaseous state is characterized by the following physical properties.
- Shape and volume : Gases neither has definite shape nor definite volume. They assume volume and shape of the container.
- Compressibility : Gases can be compressed by the application of external pressure.
- Homogeneous nature : Gases have similar composition in all parts and are therefore, homogeneous in nature.
- Density : Gases have much lower density than the solids and liquids.
- Pressure : Gases exert pressure equally in all directions.
- Liquefication : Gases can be liquefied by cooling and by applying pressure.
- Diffusion : Gases intermix readily and completely in all proportions without any mechanical aid.
FAQ – Gas & its gaseous state
Here are some important questions with answers from the Gas and gaseous state topic.
CO2 is heavier than N2 and O2 gases present in the atmosphere. But it does not form the lower layer of the atmosphere. why?
Gases possess the property of diffusion which is independent of the force of gravitation. Due to diffusion, gases mix with each other and remain almost uniformly distributed in the atmosphere.
What are the important measurable properties of gases?
The important measurable properties of gases are mass (amount), volume, pressure, temperature, viscosity, and specific heat.
What is atmospheric pressure?
The earth is surrounded by an approximately 800 km thick blanket of air. The air is pulled towards the surface by gravity and therefore, it exerts pressure on the earth’s surface. The pressure exerted by the gases of the atmosphere on the surface of the earth is called atmospheric pressure.
Define a standard pressure of one atmosphere (1 atm)?
A standard pressure of one atmosphere (1 atm) is defined as the pressure that will support a column of mercury of 76 cm height at 0°C.
Different units for atmospheric pressure and their relationships
1 atm = 76.0 cm of mercury (cm Hg) = 760 mm of mercury (mm Hg) = 760 torr.
[One atmosphere is also referred to as 760 torr. This torr unit is named after the name of Italian scientist Torricelli, who invented the barometer.]
1 atm = 1.01325 × 105 Pa = 101.325 kPa
Note: bar is another unit of pressure or atmospheric pressure.
Measurement of Atmospheric Pressure
It is measured by an instrument known as barometer.
Temperature and pressure
The properties of a gas depend upon the temperature and pressure. Hence, it is convenient to specify a particular temperature and pressure for the comparison of different gases.
S.T.P. or N.T.P
The standard conditions of temperature and pressure are abbreviated as S.T.P. or N.T.P. meaning standard temperature and pressure or normal temperature and pressure respectively.
These are: Standard temperature and pressure (S.T.P. or N.T.P.)
Temperature = 0°C or 273.15 K
Pressure = 1 atm or 760 mm Hg or 760 torr or 101.325 kPa (SI units) or 1 bar
Thus, STP denotes 0°C (or 273.15 K) temperature and 1 atm (101.325 kPa) pressure.
