Salts – fundamentals
What are salts?
Salts are formed when acids react with metals, bases, or carbonates. Most salts contain a positive metal ion and a negative ion composed of one or two non-metals.
common properties of Salts
Salts have properties in common.
- Salts are ionic compounds
- Salts have high melting points and boiling points
- Salts are electrolytes
- Salts are often soluble in water.
Commonly occurring salts
The best-known salt is sodium chloride, NaCl, which is often called ‘common salt’. Many ores and minerals are composed of salts. These include chalk and limestone (calcium carbonate), gypsum (calcium sulfate) and iron pyrites (a mixture of copper sulfide and iron sulfide).
Salt crystals, like those of sodium chloride, are often formed by crystallization from an aqueous solution. When this happens, water molecules sometimes form part of the crystal structure. This occurs in Epsom Salts
(MgSO4.7H2O), gypsum (CaSO4.2H2O) and washing soda (Na2CO3.10H2O).
The water which forms part of the crystal structure is called water of crystallization. Salts containing water of crystallization are called hydrates or hydrated salts.
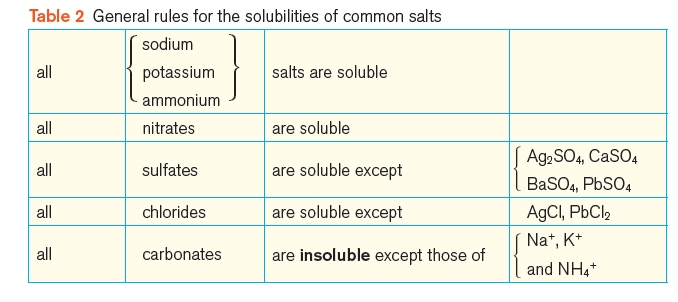
Soluble and insoluble salts
It is useful to divide salts into two categories – soluble and insoluble.
Salts with a solubility greater than 1 g per 100 g water are classed as soluble; salts with a solubility less than 1 g per 100 g water are classed as insoluble.
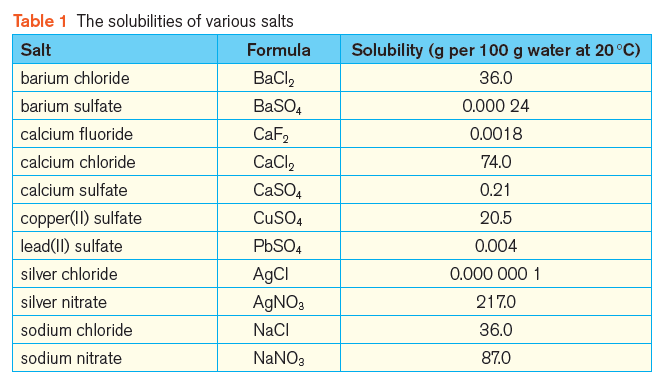
Table 2 summarises the general rules for the solubilities of common salts.