Polar and Nonpolar Covalent Bonds – concepts
In this post, we will briefly discuss the concepts of Polar and Nonpolar Covalent Bonds.
Polar Covalent bond
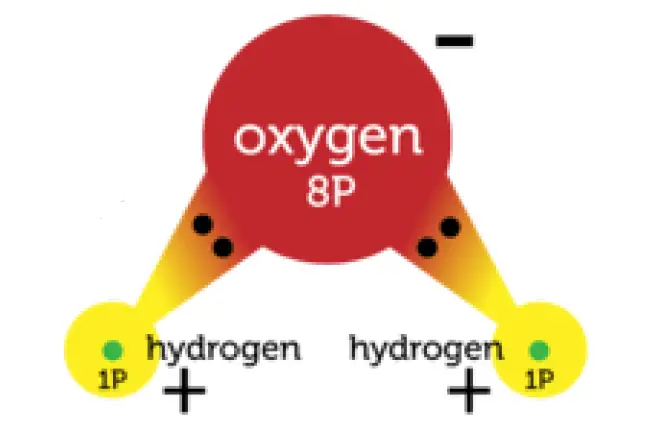
In some covalent bonds, electrons are not shared equally between the two atoms. These are called polar bonds. Figure 1 shows this polar bond for water.
Polar Covalent bonds in a water molecule
In a water molecule, the oxygen atom attracts the shared electrons more strongly because its nucleus has more positively charged protons. As a result, the oxygen atom becomes slightly negative in charge. The hydrogen atoms attract the electrons less strongly. They become slightly positive in charge.
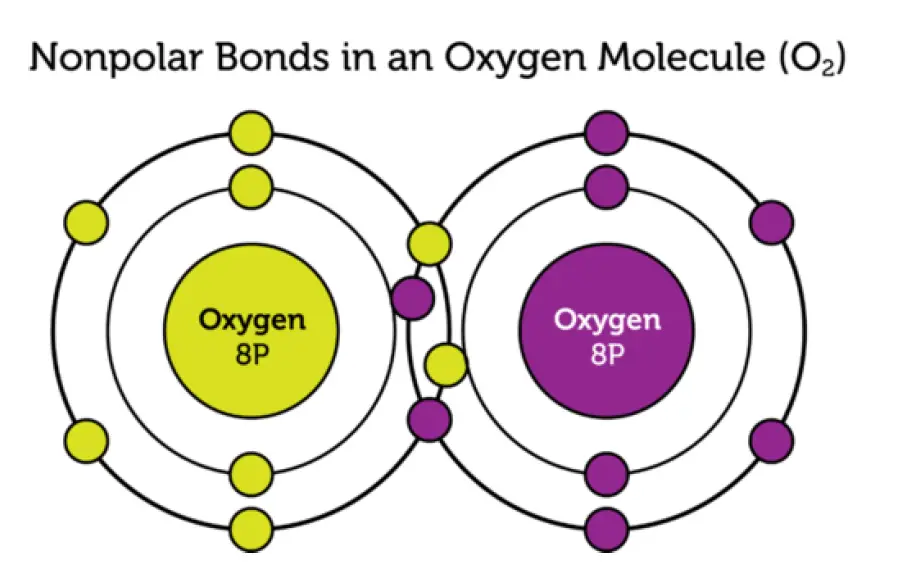
Nonpolar Covalent Bonds
In other covalent bonds, electrons are shared equally. These bonds are called nonpolar bonds. Neither atom attracts the shared electrons more strongly. As a result, the atoms remain neutral. Figure 2 shows an Oxygen molecule as an example of nonpolar bonds.
Nonpolar Covalent Bonds in an Oxygen molecule
An oxygen molecule has two nonpolar bonds. This is called a double bond. The two oxygen atoms attract equally the four shared electrons.