How does carbon bond with other atoms?
Carbon has a number of unique properties that influence how it behaves and how it bonds with other atoms:
1 ) Carbon has four valence electrons. Carbon can thus form a maximum of four bonds with other atoms as depicted in Figure 1.
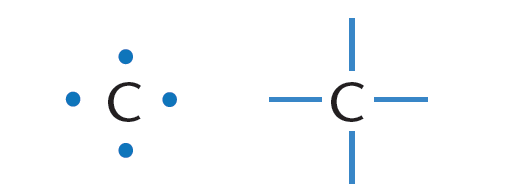
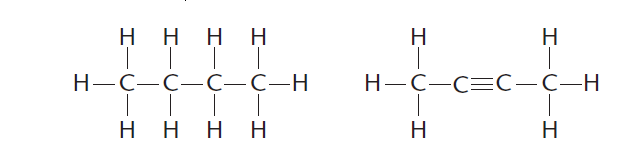
2 ) Carbon can bond strongly with other carbon atoms forming single (—), double (=) or triple (≡) bonds as shown in Figure 2.
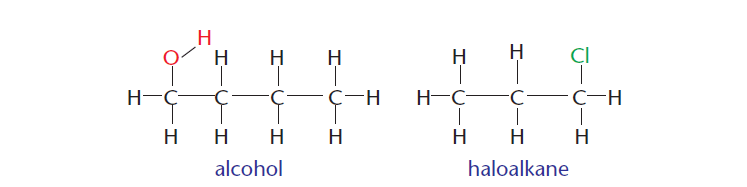
3 ) Carbon can also form bonds with other atoms such as hydrogen, oxygen, nitrogen, and halogens. Figure 3 show two organic compounds. The first one contains a hydroxyl ion (alcohol), and the second one contains a halogen (haloalkane).
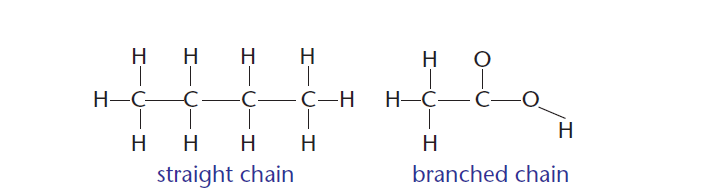
4 ) Carbon can bond to form straight and branched molecules, as shown in Figure 4.