Doping of semiconductors – revision notes
Last updated on January 27th, 2022 at 09:55 pm
Doping is the process of adding impurity atoms to an intrinsic semiconductor material or semiconductor. Here, in this post, we will discuss the doping process in detail.
semiconductors
Germanium and silicon are examples of semiconductors. Silicon and germanium have a unique property in their electron structure. Each of these elements has four electrons in its outer shell. This allows them to form crystals. The four electrons form perfect covalent bonds with four neighboring atoms, creating a lattice.
In silicon, the naturally occurring crystalline form is a silvery, metallic-looking substance.
pure silicon crystal is nearly an insulator
In a silicon lattice, all the silicon atoms bond perfectly to four neighbors, leaving no free electrons to conduct electric current. This makes a silicon crystal an insulator rather than a conductor.
Electricity requires the flow of electrons. Electric current is the flow of electrons. The ‘free electrons’ easily move between atoms in metals which makes metals good conductors of electricity.
While a silicon crystal lattice looks metallic, they differ from metallic lattices because all of the outer electrons in a silicon crystal are involved in perfect covalent bonds, not able to move around.
We can say that a pure silicon crystal is nearly an insulator, that is, very little electricity can flow through it.
doping process can change the conductivity of silicon
Silicon and germanium in their pure state do not have very good conducting properties and are called intrinsic semiconductors. An intrinsic semiconductor is a pure semiconductor material.
A pure silicon crystal is nearly an insulator, that is, very little electricity can flow through it. But we can change the conductivity of silicon through a process called doping.
How doping is done to change the conductivity of semiconductors?
In doping, you mix a small amount of an impurity atom into the silicon or germanium crystal. Doping is the process of adding impurity atoms to the intrinsic semiconductor material or semiconductors.
There are two groups of impurity atoms used in doping semiconductor materials: Trivalent atoms (Boron and Gallium) and Pentavalent atoms (Phosphorus and Arsenic).
N-type doping
In N-type doping, an element such as phosphorus or arsenic is added to the silicon (Figure 1). Phosphorus and arsenic each have five outer electrons, so they are out of place when they get into the silicon lattice.
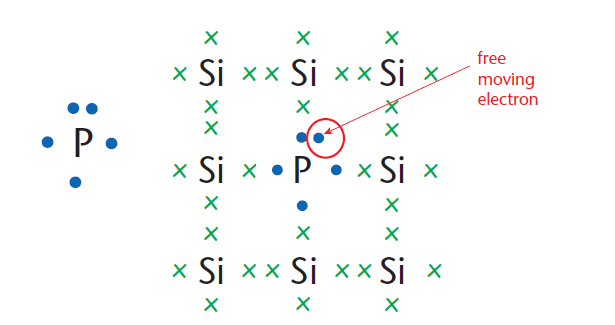
The fifth electron has nothing to bond to, therefore it is free to move around.
It only takes a small quantity of the impurity to create enough free electrons to allow an electric current to flow through the silicon. N-type silicon is a good conductor. Electrons have a negative charge, hence the name N-type.
P-type doping
With P-type doping, boron or gallium is used as the dopant. Boron and gallium each have only three electrons in their outer energy levels. When mixed into the silicon lattice, they form ‘holes’ in the lattice where a silicon electron has nothing to bond to (Figure 2).
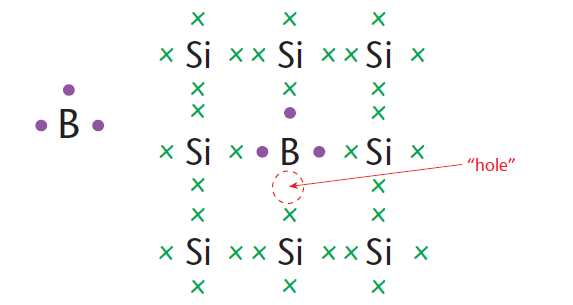
The absence of an electron creates the effect of a positive charge, hence the name P-type.
Holes can conduct current. A hole easily accepts an electron from a neighboring atom, moving the hole over a space. The P-type material is a conductor.
Takeaway | summary
Light doping of the semiconductor material into N-type or P-type turns a semiconductor crystal into a viable (but not a great) conductor. A semiconductor is a material that has the electrical conductivity between that of a conductor (metal) and an insulator (non-metal) such as glass.
A diode is the simplest semiconductor device. It allows electric current to flow in one direction but not the other. N-type and P-type silicon are not very effective when used in isolation; but when they are combined together, they yield some very interesting behavior at the junction. This is used in a diode.
