How to measure Latent Heat? | Measurement of specific latent heat
The two possible methods for measuring latent heats shown below are very similar in principle to the methods for measuring specific heat capacities. [Read about FAQs of Latent Heat]
A method for measuring the specific latent heat of vaporisation of water
The setup required for a method for measuring the latent heat of vaporization is shown in figure 1 below.
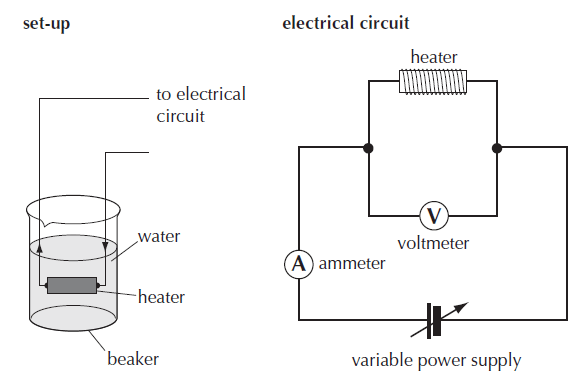
The amount of thermal energy provided to water at its boiling point is calculated using electrical energy = I t V.
The mass vaporised needs to be recorded.
The specific latent heat L= I t V /(m1 – m2)
Sources of experimental error
• Loss of thermal energy from the apparatus.
• Some water vapour will be lost before and after timing.
A method for measuring the specific latent heat of fusion of water
Provided we know the specific heat capacity of water, we can calculate the specific latent heat of fusion for water.
In the example below ice (at 0 °C) is added to warm water and the temperature of the resulting mix is measured. (figure 2)
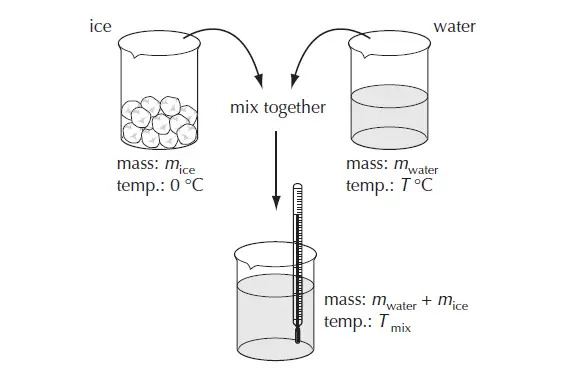
If no energy is lost from the system then,
the energy lost by water cooling down = energy gained by the ice
mwater cwater (Twater – Tmix) = mice Lfusion + mice cwater Tmix
Sources of experimental error
• Loss (or gain) of thermal energy from the apparatus.
• If the ice had not started at exactly zero, then there would be an additional term in the equation in order to account for the energy needed to warm the ice up to 0 °C.
• Water clinging to the ice before the transfer.
