Change of state – some keywords & definitions
Last updated on January 26th, 2022 at 10:12 am
In this post, we defined some keywords related to ‘change of state’ or Phase change. These words include terms like critical point, triple point, etc.
The three states of matter that you have considered are solid, liquid, and gas. Each of these states of matter can be considered as a phase.
A phase change occurs when a substance changes state – for example when ice (solid) turns to water (liquid), which then again turns to vapour.
These changes of state happen when the kinetic energy of the molecules in the substance increases or decreases.
important definitions related to change of state (phase change)
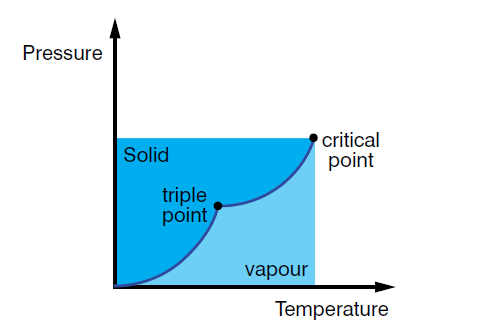
critical point – the temperature and pressure at which the liquid and gas phases of a substance become identical.
phase – the distinct form of a substance under different conditions, e.g. solid, liquid, gas phase change a change from one state of matter to another without a change in chemical composition.
A phase is a region of space in which all the physical and chemical properties of a substance are the same.
phase change diagram – a graph of pressure against temperature which can be used to show the conditions under which each phase of a substance exists.
state variable – a variable that describes the state of a dynamic system. In thermodynamics, this may include properties such as temperature, pressure, or internal energy.
triple point – the temperature and pressure at which the three phases of a substance coexist.
Melting or fusion: The change of phase from a solid to a liquid at constant temperature is called melting or fusion. This process occurs at a precise temperature (for a particular substance).
Freezing (or solidification): The change of phase from a liquid to a solid at constant temperature is called freezing (or solidification). This process occurs at a precise temperature (for a particular substance).
Boiling or by evaporation: Changing from a liquid to gas may happen by the process of boiling or by evaporation.
Evaporation can occur at any temperature (at which the substance is liquid) and it occurs only on the surface. Boiling occurs at a precise temperature throughout the liquid.
Condensation: The change of phase from gas to liquid is called condensation.
