Isotope
Last updated on December 6th, 2023 at 10:36 am
Isotopes are atoms of the same element, with different numbers of neutrons. The nuclei of different isotopes of the same element have the same number of protons but different numbers of neutrons.
Most elements have isotopes. For example, calcium has six, magnesium has three, hydrogen has three, iron has four, and chlorine has two isotopes.
Isotopes have the same chemical properties
Isotopes are atoms of the same element with different masses. All the isotopes of one element have the same number of protons. Therefore, they have the same atomic number. As isotopes have the same number of protons, they must also have the same number of electrons. This gives them the same chemical properties because chemical properties depend upon the number of electrons in an atom.
Isotopes have different physical properties
Isotopes do, however, contain different numbers of neutrons. This means that: Isotopes have the same atomic number but different mass numbers. Isotopes have different physical properties because they have different masses.
Isotopes – summary of features
Isotopes have the same:
- number of protons
- number of electrons
- atomic number
- chemical properties
Isotopes have different:
- number of neutrons
- mass number
- physical properties.
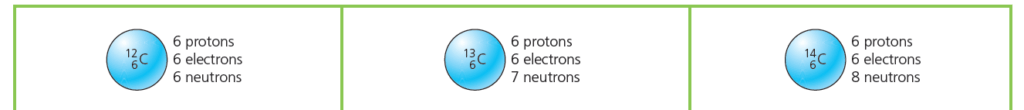
Carbon Isotopes
All carbon atoms have 6 protons. However, not all carbon atoms are identical. Some have more neutrons than others.
- Most carbon atoms are with 6 neutrons. That makes 12 nucleons (protons + neutrons) in total, so it is called carbon-12.
- But about one in every hundred carbon atoms is with 7 neutrons. It has 13 nucleons in total, so is called carbon-13.
- And a very tiny number of carbon atoms are with 8 neutrons. It has 14 nucleons in total, so is called carbon-14.
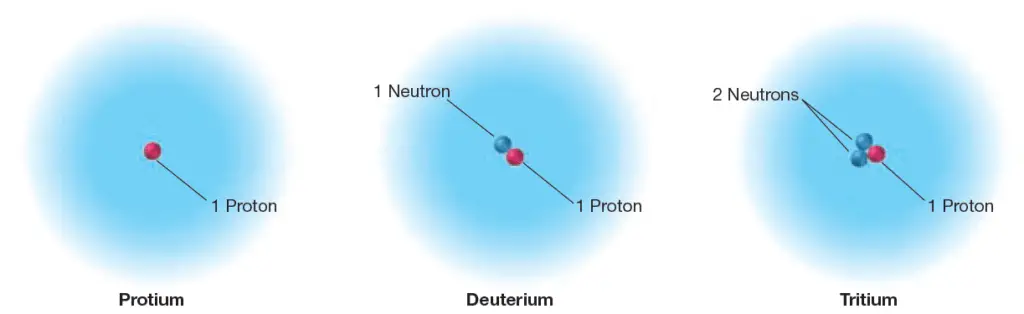
Hydrogen Isotopes
Three types of hydrogen atoms are known. The most common type of hydrogen is sometimes called protium. It accounts for 99.9885% of the hydrogen atoms found on Earth, and its nucleus consists of only a single proton.
Another type of hydrogen, deuterium, accounts for 0.0115% of Earth’s hydrogen atoms; its nucleus has one proton and one neutron.
The third form of hydrogen, tritium, has one proton and two neutrons in its nucleus. Tritium is radioactive so it is not very common at all on Earth; however, it is still hydrogen.
Some isotopes are radioactive
A carbon-14 atom behaves strangely. It is radioactive. That means its nucleus is unstable. Sooner or later the atom breaks down naturally or decays, giving out radiation in the form of rays and particles, plus a large amount of energy.
Like carbon, several other elements have radioactive isotopes – or radioisotopes – that occur naturally and eventually decay.
However, the other two isotopes of carbon (like most natural isotopes) are non-radioactive.
Use of radioisotopes
Radioisotopes are dangerous – but they are also useful. For example:
- To check for leaks – Engineers can check oil and gas pipes for leaks by adding radioisotopes to the oil or gas. If a Geiger counter detects radiation outside the pipe, it means there is a leak. Radioisotopes used in this way are called tracers.
- To treat cancer – Radioisotopes can cause cancer. But they are also used in radiotherapy to cure cancer – because the gamma rays in radiation kill cancer cells more readily than healthy cells. Cobalt-60 is usually used for this. The beam of gamma rays is aimed carefully at the site of cancer in the body.
- To kill germs and bacteria – Gamma rays kill germs too. So they are used to sterilize syringes and other disposable medical equipment. They also kill the bacteria that cause food to decay. So in many countries, foods like vegetables, fruit, spices, and meat, are treated with a low dose of radiation. Cobalt-60 and cesium-137 are used for this.
- Carbon-dating – Our bodies contain some carbon-14, taken in in food. When we die, we take no more in. But the carbon-14 atoms continue to decay. So scientists can tell the age of ancient remains by measuring the radioactivity from them. This mummy was found to be around 5300 years old.
