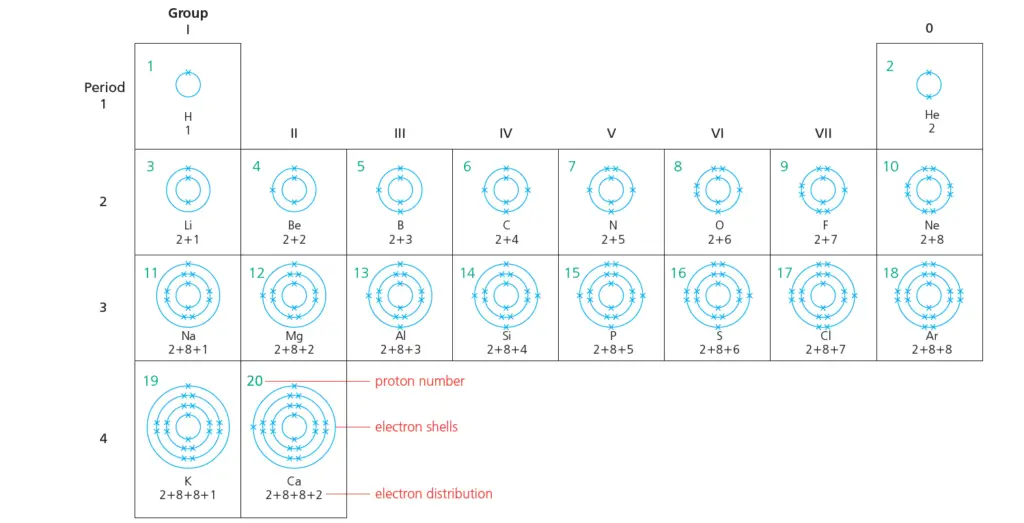
Electron shells for the first 20 elements & shells filling rules
In this post, we will present a diagram displaying the electron shells for the first 20 elements of the Periodic Table.
The number of electrons increases by 1 each time. (It is the same as the proton number.)
The shells fill according to the rules given in the next section.
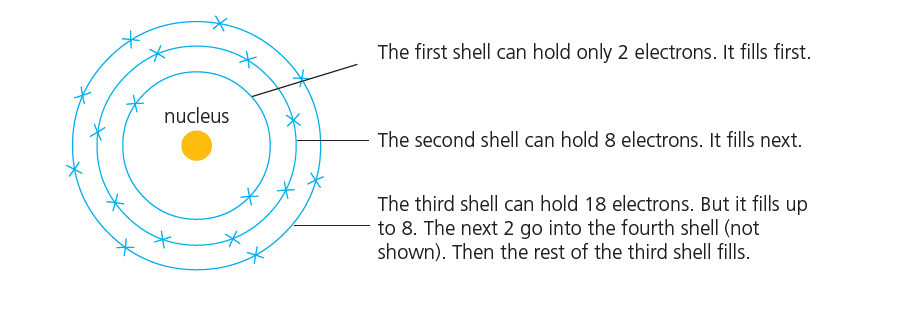
Electron shells filling rules
Electrons are arranged in shells around the nucleus.
The first shell, closest to the nucleus, is the lowest energy level. The further a shell is from the nucleus, the higher the energy level.
Each shell can hold only a certain number of electrons.
These are the rules ( Electron shells filling rules )
- The first shell can hold only 2 electrons. It fills first.
- The second shell can hold 8 electrons. It fills next.
- The third shell can hold 18 electrons. But it fills up to 8. The next 2 go into the fourth shell. Then the rest of the third shell fills.
Electron shells for the first 20 elements
The following diagram displays the electron shells for the first 20 elements of the Periodic Table.