What is titration? | How to carry out a titration?
Titration is an important method to determine the concentration of either an acid or alkali. It works by knowing the volume and concentration of one solution needed to neutralize the other. From this, you can calculate the unknown concentration.
When an acid is neutralized by a base, the pH of the solution changes. We can follow this pH change using an indicator to find out how much base just reacts with the acid to produce a neutral salt solution. This method of adding one solution from a burette to another solution in order to find out how much of the two solutions will just react with each other is called titration. When the two solutions just react and neither is in excess, we have found the endpoint of the titration.
How to carry out a titration?
In this titration experiment, we will use phenolphthalein to determine how much hydrochloric acid just reacts with 25 cm3 of sodium hydroxide solution of concentration 1 mol/dm3.
1) Measure 25.0 cm3 of sodium hydroxide solution containing 1.0 mol/dm3 (1.0 M NaOH(aq)) into a conical flask using a pipette (Figure 1).
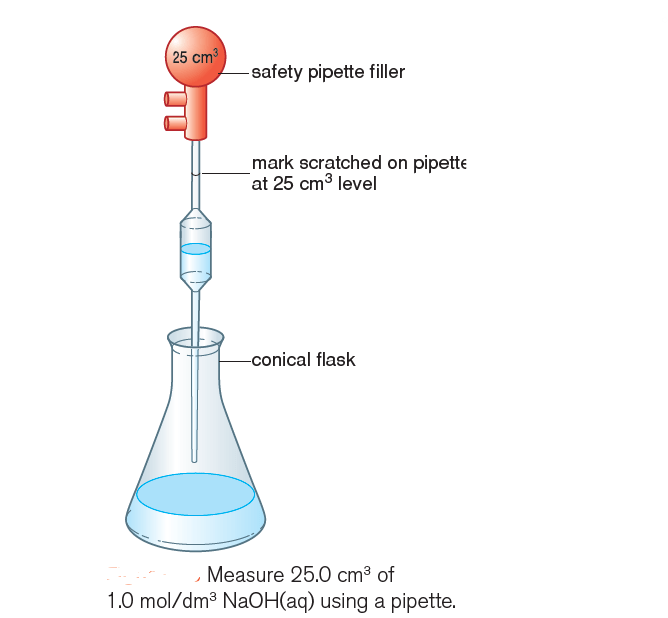
2) Add 5 to 10 drops of phenolphthalein and note the colour.
3) Add 5.00 cm3 of 1.0 mol/dm3 hydrochloric acid from a burette (Figure 2), mix well and record the colour again.
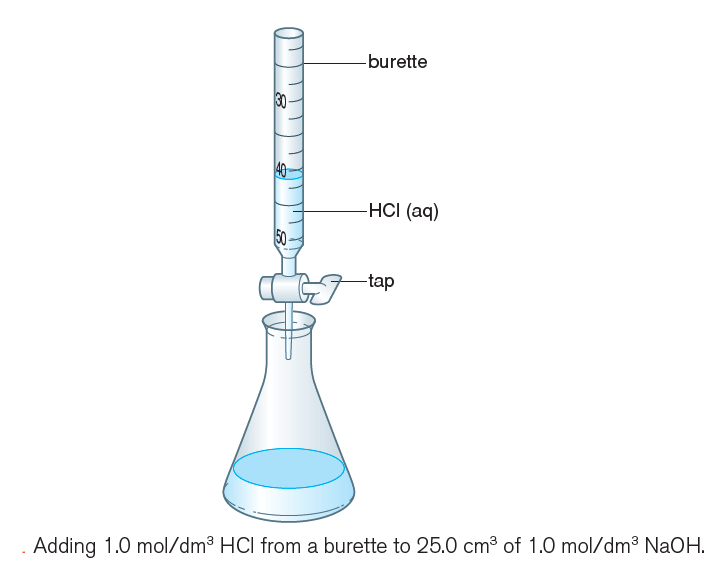
4) Record the colour also when 10.00, 15.00 and 20.00 cm3 of hydrochloric acid have been added.
5) Now add 1.00 cm3 of hydrochloric acid and record the colour again.
Repeat the addition of 1.00 cm3 nine more times and note the colour each time.
Table 1 shows the results that we should get.
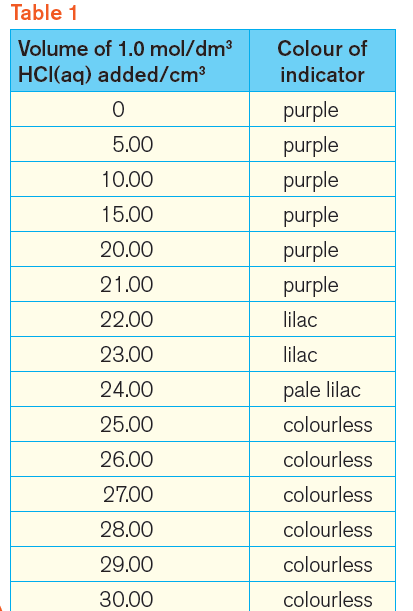
Notice that the indicator is colourless when 25.00 cm3 of hydrochloric acid has been added.
So, 25.00 cm3 of 1.0 mol/dm3 hydrochloric acid just neutralise 25.0 cm3 of 1.0 mol/dm3 sodium hydroxide.
So, 0.025 mol of HCl react with 0.025 mol of NaOH
⇒ 1 mol of HCl reacts with 1 mol of NaOH
Thus, the Titration experiment is carried out.
