How kinetic theory of gases explains the gas laws?
The kinetic theory can explain the behavior of gases. The kinetic theory of gases explains the gas laws, as well. Here, we will study a few of such explanations.
a) How kinetic theory of gases explains the Cause of gas pressure?
All the molecules in a gas are in rapid random motion, with a wide range of speeds, and repeatedly hit and rebound from the walls of the container in huge numbers per second. At each rebound, a gas molecule undergoes a change of momentum which produces a force on the walls of the container. The average force and hence the pressure they exert on the walls is constant since pressure is the force on the unit area.
b) How kinetic theory of gases explains Boyle’s law?
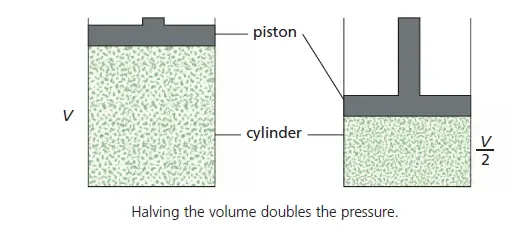
If the volume of a fixed mass of gas is halved by halving the volume of the container (Figure 1), the number of molecules per cm3 will be doubled. There will be twice as many collisions per second with the walls, i.e. the pressure is doubled. This is Boyle’s law. (Pressure is inversely proportional with volume when the temperature is constant)
c) How kinetic theory of gases explains Charles’ law?
When a gas is heated and its temperature rises, the average speed of its molecules increases. If the pressure of the gas is to remain constant, the volume must increase so that the frequency of collisions does not go up.
This is what Charles’ law states. (At constant pressure, the volume of a given mass of a gas is directly proportional to the temperature.)
d) How kinetic theory of gases explains pressure law or Gay Lussac’s law?
When a gas is heated and its temperature rises, the average speed of its molecules increases. If the volume of the gas stays constant, its pressure increases because there are more frequent and more violent collisions of the molecules with the walls.
This is what pressure law or Gay Lussac’s law states. (At constant volume, the pressure of a given mass of a gas is directly proportional to the temperature.)
