Molecular theory of solids, liquids, and gases to explain their shape and volume
The molecular theory of solids, liquids, and gases explains the reason behind their shape and volume. Let’s find out how in the next paragraph.

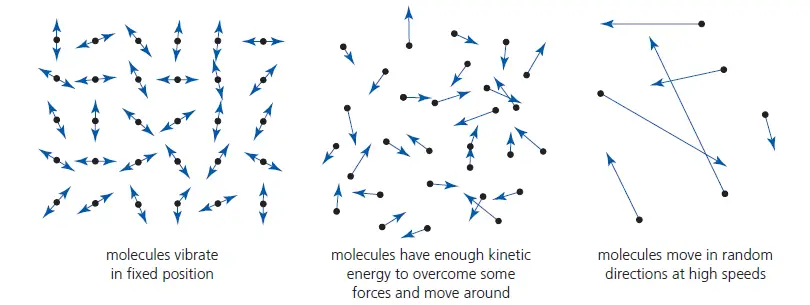
– In solids, the molecules (or atoms, or ions) are held close together by strong forces, usually in regular patterns. The molecules vibrate about their mean positions. See Figure 2 (left one is for solid)
– In liquids, the molecules still vibrate, but the forces between some molecules are overcome, allowing them to move around a little. The molecules are still almost as close together as in solids, but there is little or no regularity in their arrangement, which is constantly changing. See Figure 2 (the middle one is for liquid).
– In gases, the molecules are much further apart than in solids and liquids, and the forces between them are very, very small and usually negligible (except when they collide). This results in all molecules moving independently in random directions with a range of different (usually fast) speeds. These speeds are continually changing as a result of collisions. See Figure 2 (the rightmost one is for gases).
