How to explain the anomalous expansion of water in simple words?
Last updated on April 14th, 2021 at 02:37 pm
In the case of fluids, with the rise of temperature, the volume increases. Therefore, the density decreases. But, water expansion shows an anomalous behavior for a specific temperature zone. As we explain this behavior we will get to know why water has its maximum density (and hence minimum volume) at 4°C.
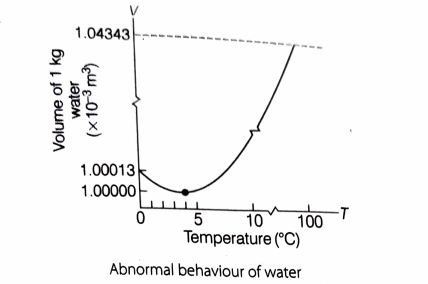
Heating of water: Water contracts on heating between the temperature 0°C to 4°C. That means when the temperature of water increases from 0°C to 4°C, its volume decreases. As a result, the density of water increases as its temperature rises from 0°C to 4°C.
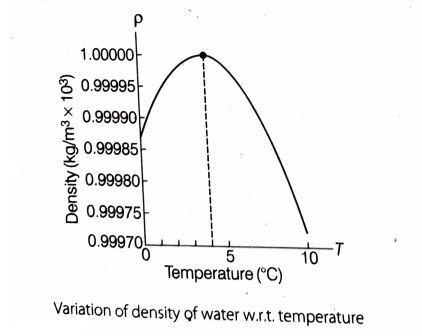
Cooling of water: When water is cooled below the room temperature (i.e., normal temperature), the volume of a given amount of water decreases (and density increases) until its temperature reaches 4°C. It’s like other fluids.
But Below 4°C, water exhibits anomalous behavior. Below 4°C the volume of water increases (and hence density decreases).
So, it is clear that water has its maximum density (and hence minimum volume) at 4°C.
