Isotope worked example
Three stable isotopes of oxygen are
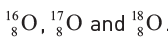
Give the number of (i) protons, (ii) nucleons, and (iii) neutrons in each isotope.
Answer:
Oxygen-16
i number of protons Z = 8
ii number of nucleons A =16
iii number of neutrons = A – Z = 8
Oxygen-17
i number of protons Z = 8
ii number of nucleons A =17
iii number of neutrons = A – Z = 9
Oxygen-18
i number of protons Z = 8
ii number of nucleons A =18
iii number of neutrons = A – Z = 10
