Which substances conduct electricity?
Last updated on March 14th, 2022 at 01:29 pm
Some substances do not conduct electricity at all; others conduct electricity in some circumstances, but not in others. In this post, we will see which solids conduct electricity. Also, we will see which liquids conduct electricity.
Which solids conduct electricity?
The only common solids which conduct electricity are metals and graphite.
When metals and graphite conduct electricity, electrons flow through the material, but there is no chemical reaction.
No solid compounds conduct electricity.
how to test whether a solid conducts electricity?
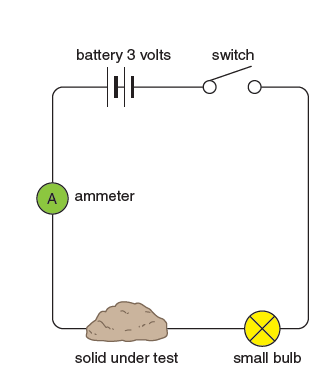
The apparatus in Figure 1 can be used to test whether a solid conducts electricity. If the solid conducts, the small electric bulb will glow when the switch is closed.
Which liquids conduct electricity?
Pure water does not readily conduct electricity. But, water containing a little sulfuric acid will readily conduct electricity. Unlike metals, the dilute sulfuric acid changes when it conducts electricity. It is decomposed, forming hydrogen and oxygen.
Electricity is a form of energy like heat. We can use it to boil water, to cook food, and to cause chemical reactions.
The decomposition of a substance, such as dilute sulfuric acid, by electricity is called electrolysis.
The compound which is decomposed is called an electrolyte and we say that it has been electrolyzed. Compounds that don’t conduct electricity and don’t get decomposed are called non-electrolytes.
how to test whether a liquid conducts electricity?
Figure 2 shows how we can test the conductivity of liquids.
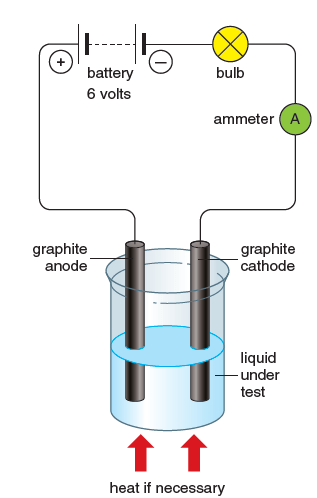
The terminals through which the current enters and leaves the electrolyte are called electrodes.
The electrode connected to the positive terminal of the battery is positive itself and is called the anode. The electrode connected to the negative terminal of the battery is negative itself and is called the cathode. If the liquid under test conducts, the bulb glows.
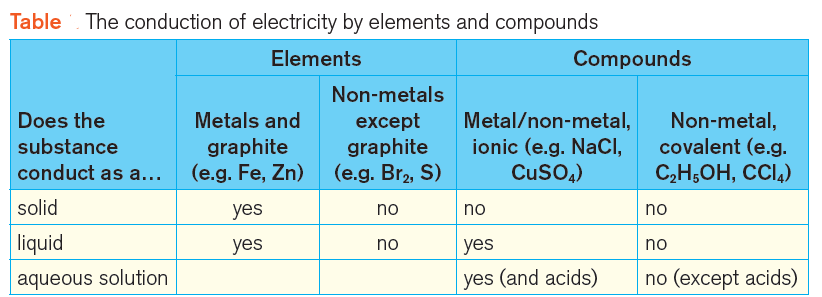
FAQ | Findings (from tests)
1 ) Do the metals and graphite (e.g. Fe, Zn) conduct as a solid?
Answer: Yes
2) Do the metals and graphite (e.g. Fe, Zn) conduct as a liquid? | Do the liquid metals conduct electricity?
Answer: Yes
3 ) Do the Non-metals except graphite (e.g. Br2, S) conduct as a solid?
Answer: No
4) Do the Non-metals except graphite (e.g. Br2, S) conduct as a liquid? | Do the liquid non-metals conduct electricity?
Answer: No
5) Do the compounds containing both metals and non-metals (ionic compounds) conduct electricity: (i) when liquid, (ii) in aqueous solution?
Answer: Ionic metal/non-metal compounds conduct electricity when they are molten (liquid) and when they are dissolved in water (aqueous). These compounds are decomposed during electrolysis.
6) Do the compounds containing only non-metals (covalent compounds) conduct: (i) when liquid, (ii) in aqueous solution?
Answer: Covalent non-metal compounds do not conduct in the liquid state or in an aqueous solution (except aqueous solutions of acids).
The answers to these questions are summarised in the Table below. (figure 3)