What is the photoelectric effect?
If high-frequency UV light radiation is shone onto the surface of a metal, it will instantly eject electrons (see Figure 1). For most metals, the necessary frequency falls in the ultraviolet range.
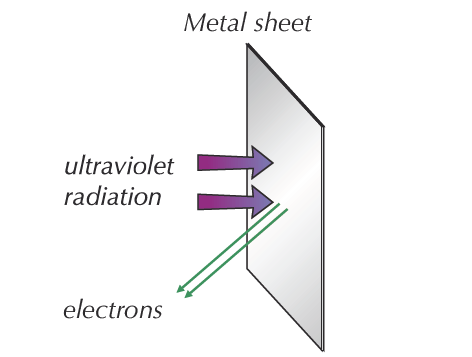
Because of the way atoms are bonded together in metals, metals contain free electrons that are able to move about the metal. The free electrons on or near the surface of the metal absorb energy from the radiation.
Before an electron can leave the surface of the metal, it needs enough energy to break the bonds holding it there. This energy is called the work function energy (f) and its value depends on the metal. If an electron absorbs this amount of energy (or more), it is released. This is called the photoelectric effect and the electrons emitted are called photoelectrons.
From photoelectric experiments, scientists came up with these conclusions:
Conclusion 1: For a given metal, no photoelectrons are emitted if the radiation has a frequency below a certain value – called the threshold frequency.
Conclusion 2: The photoelectrons are emitted with a variety of kinetic energies ranging from zero to some maximum value. This value of maximum kinetic energy increases with the frequency of the radiation but is independent of the intensity of the incident radiation.
Conclusion 3: The number of photoelectrons emitted per second is directly proportional to the intensity of the radiation.
Explaining the photoelectric effect
According to the photon model, when EM radiation hits a metal, the metal’s surface is bombarded by photons.
If one of these photons collides with a free electron, there is a one-to-one interaction between the photon and the surface electron.
The electron gains energy equal to hf (as all of the photon’s energy is transferred to the electron).
This idea could be used to explain the conclusions from the photoelectric effect, meaning that the photoelectric effect supported the particulate (particle-like) nature of EM radiation.
