Aufbau Principle – how an electron enters subshell
Aufbau Principle: An electron enters the subshell that has the least energy. The subshells are filled in the increasing order of energy.
The ascending order is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d,….
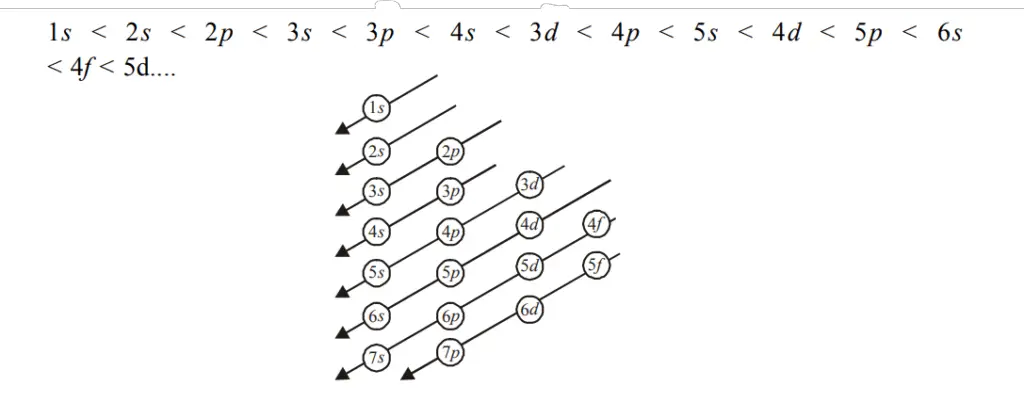
Order of filling the subshells: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d….
