Define stoichiometry
In this post, we will briefly define stoichiometry. Then we will see how to Relate quantities in a balanced chemical reaction on a molecular basis.
Define stoichiometry
The relating of one chemical substance to another using a balanced chemical reaction is called stoichiometry.
Using stoichiometry is a fundamental skill in chemistry; it greatly broadens our ability to predict what will occur and, more importantly, how much is produced in a chemical reaction.
Relate quantities in a balanced chemical reaction on a molecular basis
For example, suppose we need to know how many molecules of oxygen are needed to react with 16 molecules of H2. We can find this easily using the conversion factor derived directly from the coefficients in the balanced chemical equation. This conversion factor is derived by relating quantities in a balanced chemical reaction on a molecular basis.
For example, consider the following chemical equation:
2H2(g) + O2(g) → 2H2O(ℓ)
We can interpret this as, literally, “two hydrogen molecules react with one oxygen molecule to make two water molecules.”
That interpretation leads us directly to some equivalences
2 H2 molecules ⇔ 1 O2 molecule ⇔ 2 H2O molecules
These equivalences allow us to construct conversion factors:
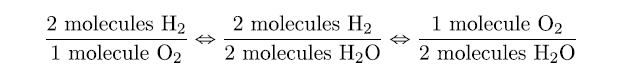
and so forth.
These conversions can be used to relate quantities of one substance to quantities of another.
For example, suppose we need to know how many molecules of oxygen are needed to react with 16 molecules of H2.
As we did with converting units, we start with our given quantity and use the appropriate conversion factor:
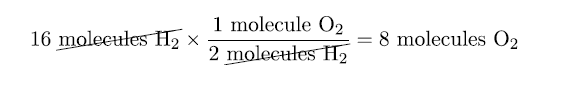
Thus using the conversion factor, we can find out that 8 molecules of O2 are needed to react with 16 molecules of H2.
Note how the unit molecules H2 cancels algebraically, just as any unit does in a conversion like this.
The conversion factor came directly from the coefficients in the balanced chemical equation. This is another reason why a properly balanced chemical equation is important.
