Molecular formulae and structural formulae of organic compounds
Chemists use a variety of ways to represent organic compounds. It is useful to know all of these so that you can recognize a molecule regardless of how it is represented. In this post, we will discuss the Molecular formulae and structural formulae of organic compounds.
Structural formulae
A structural formula is a graphic representation of the molecule that shows how every atom is bonded in an organic molecule.
The structural formula of a compound shows which atoms are attached to which within the molecule, as shown in Figure 1. Atoms are represented by their chemical symbols. Each line represents a bond in this form of representation.
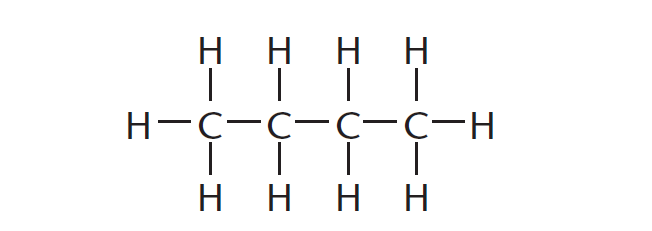
Molecular formulae
A molecular formula shows how many atoms of each type are in the organic molecule. A molecular formula representation always begins with the carbon atom starting from the left to the right! The number of atoms in the molecule is written as a subscript after the atomic symbol of each atom.
The molecular formula for the organic molecule shown in Figure 1 is C4H10. This means that this organic molecule is made up of four carbon atoms and ten hydrogen atoms.
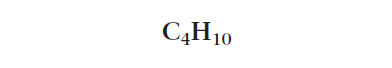
Excercise – Representing organic compounds

