Difference between Cohesion and Adhesion & their importance
Cohesion refers to the attraction of molecules for other molecules of the same kind, but Adhesion is the attraction of molecules of one kind for molecules of a different kind.
In this post, we will learn in detail what exactly Cohesion and Adhesion are, and this will help to understand the difference between them. To discuss these we will consider the cohesion and adhesion of water.
Cohesion
Cohesion refers to the attraction of molecules for other molecules of the same kind, and water molecules have strong cohesive forces thanks to their ability to form hydrogen bonds with one another.
Surface tension & Cohesion
Cohesive forces are responsible for surface tension, a phenomenon that results in the tendency of a liquid’s surface to resist rupture when placed under tension or stress.
Water molecules at the surface (at the water-air interface) will form hydrogen bonds with their neighbors, just like water molecules deeper within the liquid. However, because water molecules at the surface are exposed to air on one side, they will have fewer neighboring water molecules to bond with and will form stronger bonds with the neighbors they do have.
Surface tension causes water to form spherical droplets and allows it to support small objects, like a scrap of paper or a needle if they are placed carefully on its surface. Know more about surface tension.
Adhesion
Adhesion is the attraction of molecules of one kind for molecules of a different kind, and it can be quite strong for water, especially with other molecules bearing positive or negative charges.
capillary action and Adhesion
Adhesion enables water to “climb” upwards through thin glass tubes (called capillary tubes) placed in a beaker of water. This upward motion against gravity, known as capillary action, depends on the attraction between water molecules and the glass walls of the tube (adhesion), as well as on interactions between water molecules (cohesion).
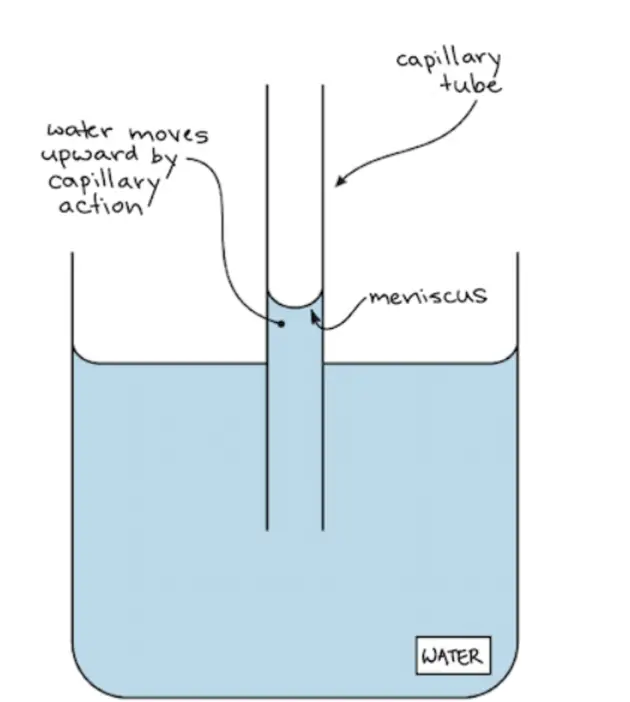
Water molecules are more strongly attracted to the glass than they are to other water molecules | meniscus
The water molecules are more strongly attracted to the glass than they are to other water molecules (because glass molecules are even more polar than water molecules). You can see this by looking at the image below: the water extends highest where it contacts the edges of the tube and dips lowest in the middle. The curved surface formed by a liquid in a cylinder or tube is called a meniscus.
Why are cohesive and adhesive forces important for life?
Cohesive and adhesive forces play a role in many water-based processes in biology, including the movement of water to the tops of trees and the drainage of tears from tear ducts in the corners of your eyes.
A simple example of cohesion in action comes from the water strider, an insect that relies on surface tension to stay afloat on the surface of the water.
