The Millikan Oil-Drop Experiment
Robert Millikan measured e, the magnitude of the elementary charge on an electron, and demonstrated the quantized nature of this charge with the help of a brilliant set of experiments from 1909 to 1913. The experiment is known as The Millikan Oil-Drop Experiment.
His apparatus, diagrammed in Figure 1, contains two parallel metallic plates. Oil droplets from an atomizer are allowed to pass through a small hole in the upper plate. Millikan used x-rays to ionize the air in the chamber so that freed electrons would adhere to the oil drops, giving them a negative charge. A horizontally directed light beam is used to illuminate the oil droplets, which are viewed through a telescope whose long axis is perpendicular to the light beam. When viewed in this manner, the droplets appear like shining stars against a dark background, and the rate at which individual drops fall can be determined.
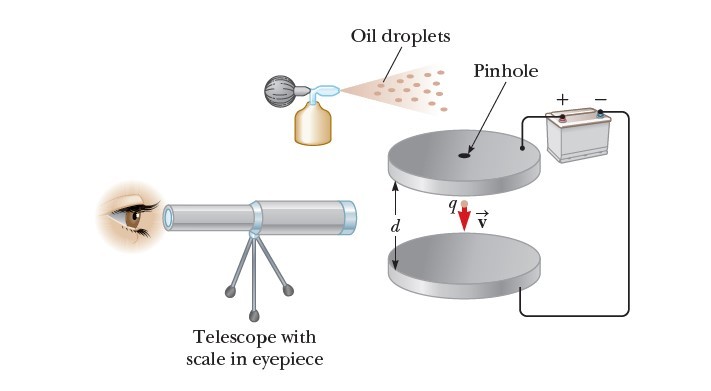
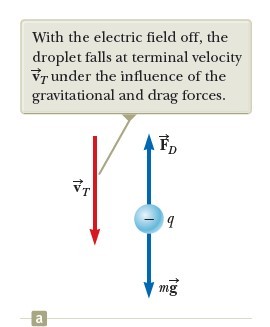
Let’s assume a single drop having a mass m and carrying a charge q is being viewed and its charge is negative. If no electric field is present between the plates, the two forces acting on the charge are the gravitational force mg acting downward and a viscous drag force FD acting upward as indicated in Figure 2a. The drag force is proportional to the drop’s speed. When the drop reaches its terminal speed VT the two forces balance each other (mg = FD).
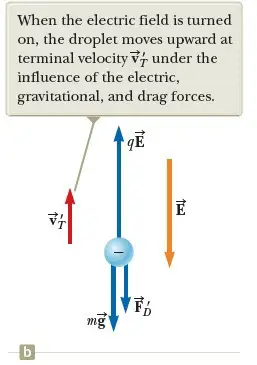
Now suppose a battery connected to the plates sets up an electric field between the plates such that the upper plate is at the higher electric potential. In this case, a third force qE acts on the charged drop. The particle in a field model applies twice to the particle: it is in a gravitational field and an electric field.
Because q is negative and E is directed downward, this electric force is directed upward as shown in Figure 2b. If this upward force is strong enough, the drop moves upward and the drag force FD’ acts downward. When the upward electric force qE balances the sum of the gravitational force and the downward drag force FD’, the drop reaches a new terminal speed VT’ in the upward direction.
With the field turned on, a drop moves slowly upward, typically at rates of hundredths of a centimeter per second. The rate of fall in the absence of a field is comparable. Hence, one can follow a single droplet for hours, alternately rising and falling, by simply turning the electric field on and off.
After recording measurements on thousands of droplets, Millikan and his coworkers found that all droplets, to within about 1% precision, had a charge equal to some integer multiple of the elementary charge e:
q = ne where n = 0, -1, -2, -3, . . .
where e = 1.6 x 10-19 C.
Millikan’s experiment yields conclusive evidence that charge is quantized. For this work, he was awarded the Nobel Prize in Physics in 1923.
